Abstract
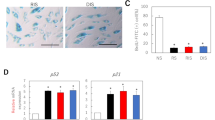
Diabetic foot ulcers (DFUs) are chronic wounds with high matrix metalloproteinase (MMP) activity, and are a frequent complication on diabetics. This work studied the expression of selected MMP and tissue inhibitor of metalloproteinases (TIMP) gene family members in DFU and normal skin biopsies, and in vitamin D-treated keratinocytes cultured from those biopsies. We report for the first time the expression of some of these genes in healthy skin. Our results suggest that vitamin D may modulate the expression of some MMP gene family members in keratinocytes. Gene expression in DFU and in non-diabetic healthy skin (control) biopsies was evaluated by RT-qPCR for MMP-1, MMP-3, MMP-8, MMP-9, MMP-10, MMP-19, TIMP-1 and TIMP-2, and also by immunohistochemistry for MMP-1 and MMP-9. Primary keratinocytes cultured from DFU and healthy skin biopsies were used for gene expression analyses of selected MMPs and TIMPs by RT-qPCR, both in the presence and absence of calcitriol. The expression of MMP-1, MMP-8, MMP-9, MMP-10, and TIMP-2 in healthy skin is reported here for the first time. DFUs showed increased MMP-1, MMP-9 and TIMP-1 expression, compared to healthy skin. Calcitriol down-regulated MMP-1 and MMP-10 expression in DFU-derived keratinocytes but not in those derived from healthy skin. Our data demonstrate the expression of certain MMPs that had not been previously described in healthy skin, and further support previous reports of MMP and TIMP up-regulation in DFUs. Our results point to calcitriol as a potential modulator for the expression of certain MMP members in DFUs.






Similar content being viewed by others
References
Bahar-Shany K, Ravid A, Koren R (2010) Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol 222(3):729–737
Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM (2000) Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am J Pathol 157(2):525–535
Bikle DD, Oda Y, Xie Z (2005) Vitamin D and skin cancer: a problem in gene regulation. J Steroid Biochem Mol Biol 97(1–2):83–91
Brem H, Tomic-Canic M (2007) Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117(5):1219–1222
Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ (1999) Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg 230(2):260–265
Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, Wilkinson RJ, Martineau AR (2009) 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology 127(4):539–548
Chen D, Li Y, Dai X, Zhou X, Tian W, Zhou Y, Zou X, Zhang C (2013) 1,25-Dihydroxyvitamin D3 activates MMP13 gene expression in chondrocytes through p38 MARK pathway. Int J Biol Sci 9(6):649–655
Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A (2012) Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 61(11):2937–2947
Durant S, Duval D, Homo-Delarche F (1986) Factors involved in the control of fibroblast proliferation by glucocorticoids: a review. Endocr Rev 7(3):254–269
Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, Krieg T, Shannon JD, Fox JW (2010) Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res 9(9):4758–4766
Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H (2003) Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem 278(42):40764–40770
Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, Conserva F, Casagrande V, Menghini R, Pontrelli P, Arisi I, D’Onofrio M, Lauro D, Khokha R, Accili D, Pugliese G, Gesualdo L, Lauro R, Federici M (2013) Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med 5(3):441–455
Garach-Jehoshua O, Ravid A, Liberman UA, Reichrath J, Glaser T, Koren R (1998) Upregulation of the calcium-dependent protease, calpain, during keratinocyte differentiation. Br J Dermatol 139(6):950–957
Gill SE, Pape MC, Khokha R, Watson AJ, Leco KJ (2003) A null mutation for tissue inhibitor of metalloproteinases-3 (Timp-3) impairs murine bronchiole branching morphogenesis. Dev Biol 261(2):313–323
Gooyit M, Peng Z, Wolter WR, Pi H, Ding D, Hesek D, Lee M, Boggess B, Champion MM, Suckow MA, Mobashery S, Chang M (2014) A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol 9(1):105–110
Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS (2007) Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J 21(10):2580–2591
Halder SK, Osteen KG, Al-Hendy A (2013) Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod 28(9):2407–2416
Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC (1996) Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA 93(18):9748–9753
Hartenstein B, Dittrich BT, Stickens D, Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z, Angel P (2006) Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J Invest Dermatol 126(2):486–496
Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y (2009) MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 175(2):533–546
Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM (2003) Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol 121(5):997–1004
Holzheimer RG, Mannick JA (eds) (2001) Surgical treatment evidence based an problem-oriented, 2010/10/29 edn. Munich, Zuckschwerdt
Hornebeck W (2003) Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol (Paris) 51(10):569–573
Jiang JX, Chen X, Fukada H, Serizawa N, Devaraj S, Torok NJ (2013) Advanced glycation end products induce fibrogenic activity in nonalcoholic steatohepatitis by modulating TNF-alpha-converting enzyme activity in mice. Hepatology 58(4):1339–1348
Knas M, Niczyporuk M, Zalewska A, Car H (2013) The unwounded skin remodeling in animal models of diabetes types 1 and 2. Physiol Res 62(5):519–526
Kobayashi H, Asano K, Kanai K, Suzaki H (2005) Suppressive activity of vitamin D3 on matrix metalloproteinase production from cholesteatoma keratinocytes in vitro. Mediators Inflamm 4:210–215
Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, Wolf E, Aumailley M, Parks WC, Werner S (2004) Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell 15(12):5242–5254
Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA (2013) Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 13(17):2670–2681
Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS (2002) Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 10(1):26–37
Lee J, Ozcan U (2014) Unfolded protein response signaling and metabolic diseases. J Biol Chem 289(3):1203–1211
Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60(1):107–114
Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, McLennan SV (2009) Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 32(1):117–119
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H (2006) Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J Diabetes Complications 20(5):329–335
Loffek S, Schilling O, Franzke CW (2011) Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38(1):191–208
Mackay AR, Hartzler JL, Pelina MD, Thorgeirsson UP (1990) Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J Biol Chem 265(35):21929–21934
Madlener M, Parks WC, Werner S (1998) Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 242(1):201–210
Martins VL, Caley M, O’Toole EA (2013) Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res 351(2):255–268
Meephansan J, Komine M, Tsuda H, Ohtsuki M (2012) Suppressive effect of calcipotriol on the induction of matrix metalloproteinase (MMP)-9 and MMP-13 in a human squamous cell carcinoma cell line. Clin Exp Dermatol 37(8):889–896
Menghini R, Uccioli L, Vainieri E, Pecchioli C, Casagrande V, Stoehr R, Cardellini M, Porzio O, Rizza S, Federici M (2013) Expression of tissue inhibitor of metalloprotease 3 is reduced in ischemic but not neuropathic ulcers from patients with type 2 diabetes mellitus. Acta Diabetol 50(6):907–910
Motzkau M, Tautenhahn J, Lehnert H, Lobmann R (2011) Expression of matrix-metalloproteases in the fluid of chronic diabetic foot wounds treated with a protease absorbent dressing. Exp Clin Endocrinol Diabetes 119(5):286–290
Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY (2008) Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 25(4):419–426
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69(3):562–573
O’Brien PD, Hinder LM, Sakowski SA, Feldman EL (2014) ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal. doi:10.1089/ars.2013.5807
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137(6):1445–1457
Pittas AG, Dawson-Hughes B (2010) Vitamin D and diabetes. J Steroid Biochem Mol Biol 121(1–2):425–429
Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB (2010) Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 33(9):2021–2023
Preston AM, Hendershot LM (2013) Examination of a second node of translational control in the unfolded protein response. J Cell Sci 126(Pt 18):4253–4261
Rayment EA, Dargaville TR, Shooter GK, George GA, Upton Z (2008) Attenuation of protease activity in chronic wound fluid with bisphosphonate-functionalised hydrogels. Biomaterials 29(12):1785–1795
Rayment EA, Upton Z, Shooter GK (2008) Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 158(5):951–961
Saarialho-Kere UK, Crouch EC, Parks WC (1995) Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol 105(2):190–196
Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC (1993) Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 92(6):2858–2866
Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG (1994) Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest 94(1):79–88
Sadowski T, Dietrich S, Muller M, Havlickova B, Schunck M, Proksch E, Muller MS, Sedlacek R (2003) Matrix metalloproteinase-19 expression in normal and diseased skin: dysregulation by epidermal proliferation. J Invest Dermatol 121(5):989–996
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85
Stephens K, Zlotogorski A, Smith L, Ehrlich P, Wijsman E, Livingston RJ, Sybert VP (1995) Epidermolysis bullosa simplex: a keratin 5 mutation is a fully dominant allele in epidermal cytoskeleton function. Am J Hum Genet 56(3):577–585
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Sudbeck BD, Pilcher BK, Welgus HG, Parks WC (1997) Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J Biol Chem 272(35):22103–22110
Takiishi T, Gysemans C, Bouillon R, Mathieu C (2010) Vitamin D and diabetes. Endocrinol Metab Clin North Am 39(2):419–446
Tarnuzzer RW, Schultz GS (1996) Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 4(3):321–325
Terasaki K, Kanzaki T, Aoki T, Iwata K, Saiki I (2003) Effects of recombinant human tissue inhibitor of metalloproteinases-2 (rh-TIMP-2) on migration of epidermal keratinocytes in vitro and wound healing in vivo. J Dermatol 30(3):165–172
Thorand B, Zierer A, Huth C, Linseisen J, Meisinger C, Roden M, Peters A, Koenig W, Herder C (2011) Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care 34(10):2320–2322
Toriseva M, Kahari VM (2009) Proteinases in cutaneous wound healing. Cell Mol Life Sci 66(2):203–224
Vaalamo M, Leivo T, Saarialho-Kere U (1999) Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 30(7):795–802
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92(8):827–839
Wacker M, Holick MF (2013) Vitamin D–effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 5(1):111–148
Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA (1996) The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 271(17):10079–10086
Widgerow AD (2011) Chronic wound fluid–thinking outside the box. Wound Repair Regen 19(3):287–291
Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C (2011) Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol 347(1–2):106–120
World Health Organization (2013) http://www.who.int/mediacentre/factsheets/fs312/en/#
Zeng W, Tahrani A, Shakher J, Varani J, Hughes S, Dubb K, Stevens MJ (2011) Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes. J Diabetes Complications 25(6):398–404
Conflict of interest
The authors declare no conflicts of interest. All authors have read and approved the final version of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-López, N., González-Curiel, I., Treviño-Santa Cruz, M.B. et al. Expression and vitamin D-mediated regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy skin and in diabetic foot ulcers. Arch Dermatol Res 306, 809–821 (2014). https://doi.org/10.1007/s00403-014-1494-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1494-2