Abstract
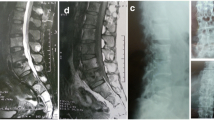
The infection with non-tuberculous mycobacterium correlates highly with immunodeficiency. Mycobacterium xenopi (M. xenopi) is most commonly isolated in the respiratory tract, as a cause of endogenous spondylodiscitis it occurs but rarely. Only seven such cases have been reported in literature. In this paper, we present the case of an about 28-year-old HIV-positive patient with a long history of back pain. MRI of the spinal column and Positron Emission Tomography with 18F-fluorodeoxyglucose as a tracer (F18-FDG-PET) confirmed the suspected spondylodiscitis. After performing a CT-controlled abscess drainage the patient’s condition improved. Because of the severe destruction of the spinal segment concerned and because of the epidural abscess formation a vertebrectomy of T10 and surgical debridement of the paravertebral soft tissue via thoracotomy became urgently necessary. The spine was stabilized by interposing a cage and an anterolateral monobar system. M. xenopi could be proven by PCR out of the intraoperative specimen. After operation and antituberculotic therapy there was a fast convalescence. Diagnostics, therapy, and clinical outcome are discussed.






Similar content being viewed by others
References
Meybeck A, Fortin C, Abgrall S, Adle-Biassette H, Hayem G, Ruimy R, Yeni P (2005) Spondylitis due to Mycobacterium xenopi in a Human Immunodeficiency Virus Type 1-infected patient: case report and review of the literature. J Clin Microbiol 43(3):1465–1466
Danesh-Clough T, Theis JC, Linden A (2000) Mycobacterium xenopi infection of the spine. Spine 1;25(5):626–628
Manfredi R, Nanetti A, Morelli S, Ferri M, Valentini R, Calza L, Chiodo F (2004) A decade surveillance study of Mycobacterium xenopi and antimicrobial susceptibility levels in a reference teaching hospital of Northern Italy: HIV-associated versus non-HIV-associated infection. HIV Clin Trials 5(4):206–215
Kulasegaram R, Richardson D, Macrae B, Ruiter A (2001) Mycobacterium xenopi osteomyelitis in a patient on highly active antiretroviral therapy (HAART). Int J STD AIDS 12:404–406
Bossert T, Bittner HB, Richter M, Rahmel A, Barten M, Gummert J, Mohr FW (2005) Successful management of two heart transplant recipients with mycobacterial pulmonary infections. Ann Thorac Surg 80(2):719–721
Telgt DS, van den Hoogen FH, Meis JF, Lemmens JA, van de Putte LB (1997) Arthritis and spondylodiscitis caused by Mycobacterium xenopi in a patient with systemic lupus erythematosus. Br J Rheumatol 36(9):1025–1026
American Thoracic Society (1997) Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 156:1–25
Schmitt H, Schnitzler N, Riehl J, Adam G, Sieberth HG, Haase G (1999) Successful treatment of pulmonary Mycobacterium xenopi infection in a natural killer cell-deficient patient with Clarithromycin, Rifabutin and Sparfloxacin. Clin Infect Dis 29(1):120–124
Bachmeyer C, Blum L, Stelianides S, Benchaa B, Gruat N, Danne O (2001) Mycobacterium xenopi pulmonary infection in an HIV infected patient under highly active antiretroviral treatment. Thorax 56:978–979
Miller WC, Perkins MD, Richardson WJ, Sexton DJ (1994) Pott’s disease caused by Mycobacterium xenopi: case report and review. Clin Infect Dis 19(6):1024–1028
Ollagnier E, Fresard A, Guglielminotti C, Carricajo A, Mosnier JF, Alexandre C, Lucht F (1998) Osteoarticular Mycobacterium xenopi infection. Presse Med 27(17):800–803
Jones PG, Schrager MA, Zabransky RJ (1995) Pott’s disease caused by Mycobacterium xenopi. Clin Infect Dis 21(5):1352
Astegneau P, Desplaces N, Vincent V, Chicheportiche V, Botherel A, Maugat S, Lebascle K, Leonard P, Desenclos J, Grosset J, Ziza J, Brucker G (2001) Mycobacterium xenopi spinal infections after discovertebral surgery: investigation and screening of a large outbreak. Lancet 1;358(9283):747–751
Kerbiriou L, Ustianowski A, Jonson MA, Gillespie SH, Miller RF, Lipman CI (2003) Human immunodeficiency virus type 1-related pulmonary Mycobacterium xenopi infection: a need to treat? Clin Infect Dis 1;37(9):1250–1254
Schwabacher H (1959) A strain of mycobacterium isolated from skin lesions of a cold blooded animal, Xenopus laevis, and its relation to acid fast bacilli occuring in man. J Hyg 44:378–380
Marks J, Schwabacher H (1965) Infection due to Mycobacterium xenopi. BMJ 1:32–33
Juffermans NP, Verbon A, Danner SA, Kuijper EJ, Speelman P (1998) Mycobacterium xenopi in HIV-infected patients: an emerging pathogen. AIDS 12:1661–1666
El-Helou P, Rachlis A, Fong I (1997) Mycobacterium xenopi infections in patients with human immunodeficiency virus infection. Clin Infect Dis 25:206–210
Prosser AJ (1986) Spinal infection with Mycobacterium xenopi. Tubercle 67:229–232
Rahman MA, Phongsathom V, Hughes T, Bielawska C (1992) Spinal infection by Mycobacterium xenopi in a non-immunosuppressed patient. Tuber Lung Dis 73:392–395
Arasteh KN, Cordes C, Ewers M (2000) HIV related NTM infection: incidence, survival analysis and associated risk factors. Eur J Med Res 5:424–430
Moon M, Woo Y, Lee K, Ha K, Kim S, Sun D (1995) Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine 20:1910–1916
Oga M, Arizono T, Takasita M, Sugioka Y (1993) Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Spine 18:1890–1894
Costrini AM, Manler DA, Gross WM, Hawkins VE, Yesner R, D’Esporo ND (1981) Clinical and roentgenographic features of nosocomial pulmonary disease due to Mycobacterium xenopi. Am Rev Respir Dis 123:104–109
Hoffner SE (1994) Pulmonary infections caused by less frequently encountered slow-growing environmental mycobacteria. Eur J Clin Microbiol Infect Dis 13:937–941
Research Committee of the British Thoracic Society (2001) First randomised trial of treatments for pulmonary disease caused by M. avium intracellulare, M. malmoense, and M. xenopi in HIV negative patients: rifampicin, ethambutol, and isoniazid versus rifampicin and ethambutol. Thorax 56:167–172
Banks J, Hunter AM, Campbell IA (1984) Pulmonary infection with Mycobacterium xenopi: review of treatment and response. Thorax 39:376–382
Griffith DE, Wallace RJ (1996) New developments in the treatment of nontuberculous mycobacterial disease. Semin Respir Iinfect 11:301–310
Alangaden GJ, Lerner SA (1997) The clinical use fluoroquinolones for the treatment of mycobacterial diseases. Clin Infect Dis 25:1213–1221
Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, von Schulthess GK (2002) FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR 179:1151–1157
Risse JH, Grünwald F, Gassel F, Biersack HJ, Schmitt O (2001) Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J 10:534–539
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobottke, R., Zarghooni, K., Seifert, H. et al. Spondylodiscitis caused by Mycobacterium xenopi . Arch Orthop Trauma Surg 128, 1047–1053 (2008). https://doi.org/10.1007/s00402-007-0553-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0553-y