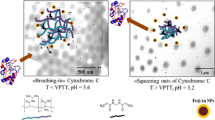
Abstract
We report on the effect of different stimuli-responsive polymer shells on Fe@Au core-shell nanoparticles (NPs) with respect to thermoresponse as well as loading and release characteristics. The hybrid NP systems were investigated using a wide array of characterization techniques including dynamic light scattering, electrophoretic mobility, UV-visible spectroscopy, and scanning transmission electron microscopy. Three different polymeric shells were selected for loading and release of l-dopa: thiolated polyethylene glycol (PEG), poly(N-isopropylacrylamide-co-acrylic acid) (PNIPAAM_AAc) microgel crosslinked with N,N′-methylenebis(acrylamide) (BIS), and finally concomitant PEG and PNIPAAM_AAc microgel (Fe@Au_PEG_Microgel). All three shells were found to exhibit high loading (∼10%) and encapsulation efficiencies up to 100 μg l-dopa/mg. Although the loading efficiencies are comparable for the three systems, Fe@Au_PEG_Microgel has the highest release (87%) at elevated temperature and acidic conditions. The attenuated release from the PEG-based systems can be attributed to stronger dipole-dipole interactions between the carboxyl group of PEG and the amino group of l-dopa.

Graphical abstract






Similar content being viewed by others
References
Kim D et al. (2007) Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. Nanomedicine-Nanotechnology Biology and Medicine 3(4):352–352
Liu H et al. (2013) Targeted tumor computed tomography imaging using low-generation dendrimer-stabilized gold nanoparticles. Chemistry—a European Journal 19(20):6409–6416
Khlebtsov N, Dykman L (2011) Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 40(3):1647–1671
Laurent S et al. (2010) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications (vol 108, p 2064, 2008). Chem Rev 110(4):2574–2574
Abakumov MA et al. (2012) Visualization of experimental glioma C6 by MRI with magnetic nanoparticles conjugated with monoclonal antibodies to vascular endothelial growth factor. Bull Exp Biol Med 154(2):274–277
Antonelli A et al. (2011) Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine 6(2):211–223
Arsalani N, Fattahi H, Nazarpoor M (2010) Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym Lett 4(6):329–338
Haw CY et al. (2010) Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceram Int 36(4):1417–1422
Lim EK et al. (2011) pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv Mater 23(21):2436
Thomas CR et al. (2010) Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J Am Chem Soc 132(31):10623–10625
Fan Z et al. (2012) Multifunctional plasmonic shell-magnetic core nanoparticles for targeted diagnostics, isolation, and photothermal destruction of tumor cells. ACS Nano 6(2):1065–1073
Zhou T, Wu BY, Xing D (2012) Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J Mater Chem 22(2):470–477
Jafari T, Simchi A, Khakpash N (2010) Synthesis and cytotoxicity assessment of superparamagnetic iron-gold core-shell nanoparticles coated with polyglycerol. J Colloid Interface Sci 345(1):64–71
Lin J et al. (2001) Gold-coated iron (Fe@Au) nanoparticles: synthesis, characterization, and magnetic field-induced self-assembly. J Solid State Chem 159(1):26–31
Levin CS et al. (2009) Magnetic-plasmonic core-shell nanoparticles. ACS Nano 3(6):1379–1388
Bandyopadhyay S et al. (2014) Synthesis and in vitro cellular interactions of superparamagnetic iron nanoparticles with a crystalline gold shell. Appl Surf Sci 316(1):171–178
Ding Y et al. (2014) Gold nanoparticles for nucleic acid delivery. Mol Ther 22(6):1075–1083
Lystvet SM et al. (2013) Anticancer activity from gold-alpha-lactalbumin nanoconstructs? J Phys Chem C 117(5):2230–2238
Lystvet SM et al. (2013) Tunable photophysical properties, conformation and function of nanosized protein-gold constructs. RSC Adv 3(2):482–495
Schotter, J., et al., Recognition of biomolecular interactions by plasmon resonance shifts in single- and multicomponent magnetic nanoparticles. Applied Physics Letters, 2008. 93(14).
Tam JM et al. (2010) Kinetic assembly of near-IR-active gold nanoclusters using weakly adsorbing polymers to control the size. Langmuir 26(11):8988–8999
Bayati S et al. (2013) Influence of poly(ethylene glycol) block length on the adsorption of thermoresponsive copolymers onto gold surfaces. J Mater Sci 48(20):7055–7062
Volden S et al. (2012) Effect of charge density matching on the temperature response of PNIPAAM block copolymer-gold nanoparticles. J Phys Chem C 116(23):12844–12853
Liu J et al. (2014) Gold nanorods coated with a thermo-responsive poly(ethylene glycol)-b-poly(N-vinylcaprolactam) corona as drug delivery systems for remotely near infrared-triggered release. Polym Chem 5(3):799–813
Maji S et al. (2015) Thermoresponsive polymer coated gold nanoparticles: from MADIX/RAFT copolymerization of N-vinylpyrrolidone and N-vinylcaprolactam to salt and temperature induced nanoparticle aggregation. RSC Adv 5(53):42388–42398
Raula J et al. (2003) Synthesis of gold nanoparticles grafted with a thermoresponsive polymer by surface-induced reversible-addition-fragmentation chain-transfer polymerization. Langmuir 19(8):3499–3504
Salmaso S et al. (2009) Cell up-take control of gold nanoparticles functionalized with a thermoresponsive polymer. J Mater Chem 19(11):1608–1615
McDonagh BH et al. (2016) l-Dopa-coated manganese oxide nanoparticles as dual MRI contrast agents and drug-delivery vehicles. Small 12(3):301–306
Lai JT, Filla D, Shea R (2002) Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 35(18):6754–6756
Xu HF, Meng FH, Zhong ZY (2009) Reversibly crosslinked temperature-responsive nano-sized polymersomes: synthesis and triggered drug release. J Mater Chem 19(24):4183–4190
Lyngso J et al. (2015) Small-angle X-ray scattering studies of thermoresponsive poly(N-isopropylacrylamide) star polymers in water. Macromolecules 48(7):2235–2243
Bekhradnia S et al. (2015) Charged star diblock copolymers in dilute solutions: synthesis, structure, and chain conformations. Macromolecules 48(8):2637–2646
Dedinaite A et al. (2010) Friction in aqueous media tuned by temperature-responsive polymer layers. Soft Matter 6(11):2489–2498
Kjoniksen AL et al. (2008) Temperature-induced formation and contraction of micelle-like aggregates in aqueous solutions of thermoresponsive short-chain copolymers. J Phys Chem B 112(11):3294–3299
Kjoniksen AL et al. (2009) Novel transition behavior in aqueous solutions of a charged thermoresponsive triblock copolymer. Colloids and Surfaces A-Physicochemical and Engineering Aspects 333(1–3):32–45
Zhu K et al. (2007) Anomalous transition in aqueous solutions of a thermoresponsive amphiphilic diblock copolymer. J Phys Chem B 111(37):10862–10870
Slomkowski S et al. (2011) Terminology of polymers and polymerization processes in dispersed systems (IUPAC recommendations 2011). Pure Appl Chem 83(12):2229–2259
Singh N, Lyon LA (2007) Au nanoparticle templated synthesis of pNIPAm nanogels. Chem Mater 19(4):719–726
Bandyopadhyay, S., et al., Incorporation of Fe@Au nanoparticles into multiresponsive pNIPAM-AAc colloidal gels modulates drug uptake and release. Colloid and Polymer Science, 2016: 1–14.
Holmberg, K., et al., Surfactants and polymers in aqueous solution. 2002: John Wiley & Sons, Ltd.
Börjesson J et al. (2007) Effect of poly(ethylene glycol) on enzymatic hydrolysis and adsorption of cellulase enzymes to pretreated lignocellulose. Enzym Microb Technol 41(1–2):186–195
Saeki S et al. (1976) Upper and lower critical solution temperatures in poly (ethylene glycol) solutions. Polymer 17(8):685–689
Karlstroem G (1985) A new model for upper and lower critical solution temperatures in poly(ethylene oxide) solutions. J Phys Chem 89(23):4962–4964
Kjellander R, Florin E (1981) Water structure and changes in thermal stability of the system poly(ethylene oxide)-water. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 77(9):2053–2077
Siepmann J, Peppas NA (2011) Higuchi equation: derivation, applications, use and misuse. Int J Pharm 418(1):6–12
Brazel CS, Peppas NA (2000) Modeling of drug release from swellable polymers. Eur J Pharm Biopharm 49(1):47–58
Acknowledgements
The authors would like to thank NorFab for the financial support in connection to the use of NTNU Nanolab and the Faculty of Natural Sciences and Technology, NTNU, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 332 kb)
Rights and permissions
About this article
Cite this article
Bandyopadhyay, S., Alvi, M.A.A., Sharma, A. et al. Influence of polymer coating on release of l-dopa from core-shell Fe@Au nanoparticle systems. Colloid Polym Sci 295, 391–402 (2017). https://doi.org/10.1007/s00396-017-4015-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4015-y