Abstract
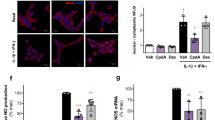
The role of adaptive immunity in myocardial recovery post myocardial infarction (MI), particularly the immune response by B lymphocytes, remains elusive. Bone marrow immune microenvironment in response to MI is remotely regulated by the hypothalamic pituitary adrenal (HPA) axis. We utilized the cardioprotective actions of SGLT2 inhibitor to identify and characterize bone marrow B cell subsets that respond to myocardial injury. Initially, we preformed ligation of left anterior descendant (LAD) coronary artery in male C57BL/6J mice to monitor the dynamic changes of immune cells across tissues. Mechanistic insights from mouse models demonstrated arrest of bone marrow B cell maturation and function 24 h post MI. A secondary MI model (twice MIs) in mice was established for the first time to evaluate the dosage-dependent cardioprotection of empagliflozin (EMPA). Single-cell RNA-Seq further demonstrated that EMPA restored bone marrow naïve B cell (B220+CD19+CD43−IgM+IgD+) counts and function. Additionally, we recruited 14 acute MI patients with single LAD disease, and profiled B cells post percutaneous coronary intervention (PCI) (compared to 18 matched no-MI controls). We revealed a positive correlation of increased B cell counts with enhanced ejection fraction in MI patients with PCI while lymphopenia was associated with patients with heart failure. Mechanistically, MI triggers the release of glucocorticoids from neuroendocrine system, inducing NHE1-mediated autophagic death of bone marrow B cells while repressing B cell progenitor proliferation and differentiation. Infusion of B cells derived from bone marrow significantly improved cardiac function and diminished infarct size post MI. These findings provide new mechanistic insights into regulation of adaptive immune response post MI, and support targeting bone marrow B cell development for improved ventricular remodeling and reduced heart failure after MI.










Similar content being viewed by others
Data availability
The RNA-seq (GSE193425) and scRNA-seq (GSE193316) datasets are available via GEO. All other data are available in the main text or the Supplementary Materials. Requests for reagents should be directed to the corresponding author.
Abbreviations
- MI:
-
Myocardial infarction
- I/R:
-
Ischemia/reperfusion
- SGLT2:
-
Sodium-glucose cotransporter 2
- scRNA-Seq:
-
Single-cell RNA sequencing
- BM:
-
Bone marrow
- EF:
-
Ejection fraction
- GR:
-
Glucocorticoids
- NHE1:
-
Na+/H+-exchanger 1
References
Adamo L, Rocha-Resende C, Lin CY, Evans S, Williams J, Dun H, Li W, Mpoy C, Andhey PS, Rogers BE, Lavine K, Kreisel D, Artyomov M, Randolph GJ, Mann DL (2020) Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight. https://doi.org/10.1172/jci.insight.134700
Adamo L, Staloch LJ, Rocha-Resende C, Matkovich SJ, Jiang W, Bajpai G, Weinheimer CJ, Kovacs A, Schilling JD, Barger PM, Bhattacharya D, Mann DL (2018) Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. https://doi.org/10.1172/jci.insight.120137
Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, Aerts S (2017) SCENIC: single-cell regulatory network inference and clustering. Nat Methods 14:1083–1086. https://doi.org/10.1038/nmeth.4463
Baartscheer A, Schumacher CA, Wust RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ (2017) Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 60:568–573. https://doi.org/10.1007/s00125-016-4134-x
Bahit MC, Kochar A, Granger CB (2018) Post-myocardial infarction heart failure. JACC Heart Fail 6:179–186. https://doi.org/10.1016/j.jchf.2017.09.015
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R (2018) Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36:411–420. https://doi.org/10.1038/nbt.4096
Calcagno DM, Ng RP Jr, Toomu A, Zhang C, Huang K, Aguirre AD, Weissleder R, Daniels LB, Fu Z, King KR (2020) The myeloid type I interferon response to myocardial infarction begins in bone marrow and is regulated by Nrf2-activated macrophages. Sci Immunol. https://doi.org/10.1126/sciimmunol.aaz1974
Cariappa A, Chase C, Liu H, Russell P, Pillai S (2007) Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood 109:2339–2345. https://doi.org/10.1182/blood-2006-05-021089
Courties G, Frodermann V, Honold L, Zheng Y, Herisson F, Schloss MJ, Sun Y, Presumey J, Severe N, Engblom C, Hulsmans M, Cremer S, Rohde D, Pittet MJ, Scadden DT, Swirski FK, Kim DE, Moskowitz MA, Nahrendorf M (2019) Glucocorticoids regulate bone marrow B lymphopoiesis after stroke. Circ Res 124:1372–1385. https://doi.org/10.1161/CIRCRESAHA.118.314518
Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE, Harvey RP (2019) Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife. https://doi.org/10.7554/eLife.43882
Goodchild TT, Robinson KA, Pang W, Tondato F, Cui J, Arrington J, Godwin L, Ungs M, Carlesso N, Weich N, Poznansky MC, Chronos NA (2009) Bone marrow-derived B cells preserve ventricular function after acute myocardial infarction. JACC Cardiovasc Interv 2:1005–1016. https://doi.org/10.1016/j.jcin.2009.08.010
Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S (2007) Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 115:1754–1761. https://doi.org/10.1161/CIRCULATIONAHA.106.653964
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Hoffmann J, Luxán G, Abplanalp WT, Glaser S-F, Rasper T, Fischer A, Muhly-Reinholz M, Potente M, Assmus B, John D, Zeiher AM, Dimmeler S (2021) Post-myocardial infarction heart failure dysregulates the bone vascular niche. Nat Commun 12:3964–3964. https://doi.org/10.1038/s41467-021-24045-4
Hofmann U, Frantz S (2015) Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 116:354–367. https://doi.org/10.1161/CIRCRESAHA.116.304072
Horckmans M, Bianchini M, Santovito D, Megens RTA, Springael JY, Negri I, Vacca M, Di Eusanio M, Moschetta A, Weber C, Duchene J, Steffens S (2018) Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation 137:948–960. https://doi.org/10.1161/CIRCULATIONAHA.117.028833
Jarcho JA (2020) More evidence for SGLT2 inhibitors in heart failure. N Engl J Med 383:1481–1482. https://doi.org/10.1056/NEJMe2027915
Jiang K, Tu Z, Chen K, Xu Y, Chen F, Xu S, Shi T, Qian J, Shen L, Hwa J, Wang D, Xiang Y (2022) Gasdermin D inhibition confers antineutrophil-mediated cardioprotection in acute myocardial infarction. J Clin Invest. https://doi.org/10.1172/JCI151268
Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, Xu S, Xu Y, Hwa J, Li J, Shang H, Xiang Y (2021) Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. https://doi.org/10.1007/s13238-020-00809-4
Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, Rim JH, Hwang I, Lee CJ, Lee M, Oh CM, Jeon JY, Gee HY, Kim JH, Lee BW, Kang ES, Cha BS, Lee MS, Yu JW, Cho JW, Kim JS, Lee YH (2020) SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 11:2127. https://doi.org/10.1038/s41467-020-15983-6
Lindsley RC, Thomas M, Srivastava B, Allman D (2007) Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood 109:2521–2528. https://doi.org/10.1182/blood-2006-04-018085
Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG (2003) Bcl11a is essential for normal lymphoid development. Nat Immunol 4:525–532. https://doi.org/10.1038/ni925
Lopaschuk GD, Verma S (2020) Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 5:632–644. https://doi.org/10.1016/j.jacbts.2020.02.004
Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Juni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S (2020) Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 141:704–707. https://doi.org/10.1161/CIRCULATIONAHA.119.044235
Monaco G, Lee B, Xu W, Mustafah S, Hwang YY, Carre C, Burdin N, Visan L, Ceccarelli M, Poidinger M, Zippelius A, Pedro de Magalhaes J, Larbi A (2019) RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep 26(1627–1640):e1627. https://doi.org/10.1016/j.celrep.2019.01.041
Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA (2019) Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37:773–782. https://doi.org/10.1038/s41587-019-0114-2
Qiu X, Hill A, Packer J, Lin D, Ma YA, Trapnell C (2017) Single-cell mRNA quantification and differential analysis with Census. Nat Methods 14:309–315. https://doi.org/10.1038/nmeth.4150
Rurik JG, Aghajanian H, Epstein JA (2021) Immune cells and immunotherapy for cardiac injury and repair. Circ Res 128:1766–1779. https://doi.org/10.1161/CIRCRESAHA.121.318005
Song S, Cao C, Choukrallah MA, Tang F, Christofori G, Kohler H, Wu F, Fodor BD, Frederiksen M, Willis SN, Jackson JT, Nutt SL, Dirnhofer S, Stadler MB, Matthias P (2021) OBF1 and Oct factors control the germinal center transcriptional program. Blood 137:2920–2934. https://doi.org/10.1182/blood.2020010175
Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, Nagareddy PR (2020) Neutrophil-Derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 141:1080–1094. https://doi.org/10.1161/CIRCULATIONAHA.119.043833
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R (2019) Comprehensive integration of single-cell data. Cell 177:1888-1902.e1821. https://doi.org/10.1016/j.cell.2019.05.031
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. https://doi.org/10.1073/pnas.0506580102
Swirski FK, Nahrendorf M (2018) Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18:733–744. https://doi.org/10.1038/s41577-018-0065-8
Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP (2005) c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity 23:275–286. https://doi.org/10.1016/j.immuni.2005.08.005
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia 61:722–726. https://doi.org/10.1007/s00125-017-4509-7
Voelkl J, Lin Y, Alesutan I, Ahmed MS, Pasham V, Mia S, Gu S, Feger M, Saxena A, Metzler B, Kuhl D, Pichler BJ, Lang F (2012) Sgk1 sensitivity of Na(+)/H(+) exchanger activity and cardiac remodeling following pressure overload. Basic Res Cardiol 107:236. https://doi.org/10.1007/s00395-011-0236-2
von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, Alber H, Berger R, Lichtenauer M, Saely CH, Moertl D, Auersperg P, Reiter C, Rieder T, Siller-Matula JM, Gager GM, Hasun M, Weidinger F, Pieber TR, Zechner PM, Herrmann M, Zirlik A, Holman RR, Oulhaj A, Sourij H (2022) Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. https://doi.org/10.1093/eurheartj/ehac494
Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu Z, Liu Z, Du J, Wang L, Yin C, Liao Y, Shang H, Martin KA, Herzog RI, Young LH, Qian L, Hwa J, Xiang Y (2018) Diabetes exacerbates myocardial ischemia/reperfusion injury by down-regulation of MicroRNA and up-regulation of O-GlcNAcylation. JACC Basic Transl Sci 3:350–362. https://doi.org/10.1016/j.jacbts.2018.01.005
Wang W, Org T, Montel-Hagen A, Pioli PD, Duan D, Israely E, Malkin D, Su T, Flach J, Kurdistani SK, Schiestl RH, Mikkola HK (2016) MEF2C protects bone marrow B-lymphoid progenitors during stress haematopoiesis. Nat Commun 7:12376. https://doi.org/10.1038/ncomms12376
Wilker PR, Kohyama M, Sandau MM, Albring JC, Nakagawa O, Schwarz JJ, Murphy KM (2008) Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nat Immunol 9:603–612. https://doi.org/10.1038/ni.1609
Wolock SL, Lopez R, Klein AM (2019) Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst 8(281–291):e289. https://doi.org/10.1016/j.cels.2018.11.005
Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M (2013) Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62:24–35. https://doi.org/10.1016/j.yjmcc.2013.04.023
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269. https://doi.org/10.1038/nm.3804
Zelniker TA, Braunwald E (2020) Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol 75:422–434. https://doi.org/10.1016/j.jacc.2019.11.031
Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z (2013) B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19:1273–1280. https://doi.org/10.1038/nm.3284
Funding
The National Key Research and Development Program of China (2017YFC1700402); The National Outstanding Youth Science Fund Project of National Natural Science Foundation of China (81822048); Fundamental Research Funds for the Central Universities (22120210162); The Frontier Science Research Center for Stem Cells, Ministry of Education.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YaX designed the study. YuX, KJ, FC, YW, CZ and DW performed the animal experiments and the in vitro experiments. YaX, YuX, KJ, KC, JQ, YY, JH, HW and BY analyzed the data. YaX wrote the manuscripts. YuX and KJ are co-first authors based on their equally contribution.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Jiang, K., Chen, F. et al. Bone marrow-derived naïve B lymphocytes improve heart function after myocardial infarction: a novel cardioprotective mechanism for empagliflozin. Basic Res Cardiol 117, 47 (2022). https://doi.org/10.1007/s00395-022-00956-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00956-1