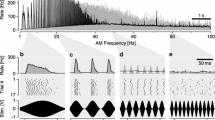
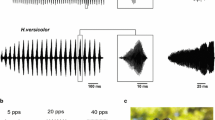
Abstract
We compare the temporal and directional processing properties of an identified auditory interneuron, ON1, between species with calling songs containing relatively low and high pulse rates (Teleogryllus oceanicus and Gryllus texensis, respectively). Using information theory, we find that the ON1 of G. texensis encodes higher amplitude-modulation frequencies than that of T. oceanicus. Bilateral differences in ON1 responses are also more pronounced in G. texensis, particularly for rapid, G. texensis-like stimuli. We show that brief silent intervals in a pulse train, such as those that occur in the natural calling song of G. texensis, enhance the representation of the stimulus pulse pattern as well as bilateral differences in activity. Our results suggest that the characteristics of an identified neuron vary, across cricket species, in accordance with the temporal structures of their communication signals.









Similar content being viewed by others
References
Atkins G, Pollack GS (1986) Age dependent occurrence of an ascending axon on the omega neuron of the cricket, Teleogryllus oceanicus. J Comp Neurol 243:527–534
Atkins G, Ligman S, Burghardt F, Stout J (1984) Changes in phonotaxis by the female cricket after killing identified acoustic interenurons. J Comp Physiol A 154:795–804
Balakrishnan R, Pollack GS (1996) Recognition of courtship song in the field cricket, Teleogryllus oceanicus. Anim Behav 51:353–366
Borst A, Theunissen FE (1999) Information theory and neural coding. Nat Neurosci 2:947–957
Brown WD (1999) Mate choice in tree crickets and their kin. Annu Rev Entomol 44:371–396
Casaday GB, Hoy RR (1977) Auditory interneurons in the cricket Teleogryllus oceanicus: physiological and anatomical properties. J Comp Physiol 121:1–13
Codon CJ, Galazyuk A, Feng AS (1997) Neurons in the auditory cortex of the little brown bat exhibit selectivity for complex-amplitude modulated signals that mimic echoes from fluttering targets insects. Audit Neurosci 3:269–287
Coro F, Pérez M, Mora E, Boada D, Conner WE, Sandeford MV, Avila H (1998) Receptor cell habituation in the A1 auditory receptor of four noctuoid moths. J Exp Biol 201:2879–2890
Doherty JA (1991) Song recognition and localization in the phonotaxis behavior of the field cricket Gryllus bimaculatus (Orthoptera, Gryllidae). J Comp Physiol A 168:213–222
Faulkes Z, Pollack GS (2000) Effects of inhibitory timing on contrast enhancement in auditory circuits in crickets (Teleogryllus oceanicus). J Neurophysiol 84:1247–1255
Givois V, Pollack GS (2000) Sensory habituation of auditory receptor neurons: Implications for sound localisation. J Exp Biol 203:2629–2537
Grace JA, Amin N, Singh NC, Theunissen FE (2003) Selectivity for conspecific song in the zebra finch auditory forebrain. J Neurophysiol 89:472–487
Gray DA, Cade WH (1999) Sex, death and genetic variation: natural and sexual selection on cricket song. Proc R Soc Lond B 266:707–709
Hedwig B, Poulet JFA (2004) Complex auditory behaviour emerges from simple reactive steering. Nature 430:781–785
Helversen D von, Rheinlaender J (1988) Interaural intensity and time discrimination in an unrestrained grasshopper: a tentative behavioural approach. J Comp Physiol A 162:333–340
Helversen D von, Helversen O von (1995) Acoustic pattern recognition and orientation in orthopteran insects: Parallel or serial processing? J Comp Physiol A 177:767–774
Horseman G, Huber F (1994) Sound localisation in crickets. II. Modeling the role of a simple neural network in the prothoracic ganglion. J Comp Physiol A 175:399–413
Krahe R, Larsen ON, Ronacher B (2000) Directional hearing is only weakly dependent on the rise time of acoustic stimuli. J Acoust Soc Am 107:1067–1070
Machens CK, Stemmler MB, Prinz P, Krahe R, Ronacher B, Herz AV (2001) Representation of acoustic communication signals by insect auditory receptor neurons. J Neurosci 21:3215–3227
Marsat G, Pollack GS (2004) Differential temporal coding of diverse acoustic signals by a single interneuron. J Neurophysiol 92:939–948
Martin SD, Gray DA, Cade WH (2000) Fine-scale temperature effects on cricket calling song. Can J Zool 78:706–712
Michelsen A (1998) Biophysics of sound localization in insects. In: Hoy R, Popper AN, Fay RR (eds) Comparative hearing: insects. Springer, Berlin Heidelberg New York
Nabatiyan A, Poulet JFA, de Polavieja GG, Hedwig B (2003) Temporal pattern recognition based on instantaneous spike rate coding in a simple auditory system. J Neurophysiol 90:2484–2493
Nagarajan SS, Cheung SW, Bedenbaugh P, Beitel RE, Scheiner CE, Merzenich MM (2002) Representation of spectral and temporal envelope of twitter vocalization in common marmoset primary auditory cortex. J Neurophysiol 87:1723–11737
Pollack GS (1986) Discrimination of calling song models by the cricket, Teleogryllus oceanicus: the influence of sound direction on neural encoding of the stimulus temporal pattern and on phonotactic behavior. J Comp Physiol A 158:549–561
Pollack GS (1998) Neural processing of acoustic signals. In: Hoy R, Popper AN, Fay RR (eds) Comparative hearing: insects. Springer, Berlin Heidelberg New York, pp 139–196
Pollack GS (2003) Sensory cues for sound localization in the cricket Teleogryllus oceanicus: interaural difference in response strength versus interaural latency difference. J Comp Physiol A 189:143–151
Pollack GS, El-Feghaly E (1993) Calling song recognition in the cricket Teleogryllus oceanicus: comparison of the effects of stimulus intensity and sound spectrum on selectivity for temporal pattern. J Comp Physiol A 171:759–766
Rice WR (1988) Analyzing tables of statistical tests. Evolution 43:223–225
Rieke F, Bodnar DA, Bialek W (1995) Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proc R Soc Lond B 262:259–265
Roddey JC, Girish B, Miller JP (2000) Assessing the performance of neural encoding models in the presence of noise. J Comput Neurosci 8:95–112
Rose GJ, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads—matched temporal filters. J Comp Physiol A 154:211–219
Rose GJ, Brenowitz EA, Capranica RR (1985) Species specificity and temperature dependency of temporal processing by the auditory midbrain of 2 species of treefrogs. J Comp Physiol A 157:763–769
Samson A-H, Pollack GS (2002) Encoding of sound localisation cues by an identified auditory interneuron: effects of stimulus temporal pattern. J Neurophysiol 88:2322–2328
Schildberger K, Hörner M (1988) The function of auditory neurons in cricket phonotaxis. I. Influence of hyperpolarization of identified neurons on sound localization. J Comp Physiol A 163:621–631
Selverston AI, Kleindienst HU, Huber F (1985) Synaptic connectivity between cricket auditory interneurons as studied by photoinactivation. J Neurosci 5:1283–1292
Sippel M, Breckow J (1984) Non-monotonic response-intensity characteristics of auditory receptor cells in Locusta migratoria. J Comp Physiol A 155:633–638
Smith SW (1997) The scientist and engineer’s guide to digital signal processing. California Technical Publishing, San Diego
Souroukis K, Cade WH, Rowell G (1992). Factors that possibly influence variation in the calling song of field cricket: temperature, time, and male size, age, and wing morphology. Can J Zool 70:950–955
Stabel J, Wendler G, Scharstein H (1989) Cricket phonotaxis: localization depends on recognition of the calling song pattern. J Comp Physiol A 165:167–177
Strausfeld NJ, Seyan HS, Wohlers D, Bacon JP (1983). Lucifer Yellow histology. In: Strausfeld NJ (ed) Functional neuroanatomy. Springer, Berlin Heidelberg New York, pp 132–155
Wagner WE Jr, Murray A-M, Cade WH (1995) Phenotypic variation in the mating preferences of female field crickets, Gryllus integer. Anim Behav 49:1269–1281
Walker TJ (2000) Pulse rates in the songs of trilling field crickets (Orthoptera: Gryllidae: Gryllus). Ann Entomol Soc Am 93:565–572
Acknowledgements
We thank David Gray and Gary Marsat for generously providing song recordings, and the two anonymous referees for their helpful comments. This work was supported by the Natural Sciences and Engineering Research Council of Canada. The experiments comply with the “Principles of animal care”, publication No. 86-23, revised 1985, of the National Institute of Health, and also with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunstall, D.N., Pollack, G.S. Temporal and directional processing by an identified interneuron, ON1, compared in cricket species that sing with different tempos. J Comp Physiol A 191, 363–372 (2005). https://doi.org/10.1007/s00359-004-0591-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-004-0591-7