Abstract
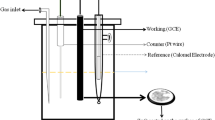
In this work, electrochemical detection of ammonia solution (NH4OH) using sol–gel-synthesized tin oxide (SnO2) nanoparticles is investigated. Structural characteristics were studied using X-ray diffraction, scanning electron microscope and energy-dispersive X-ray spectroscopy that confirmed the formation of tin oxide nanoparticles. Optical characterizations were also studied via UV–Vis–IR spectroscopy and photoluminescence spectroscopy. After successful confirmation of sol–gel synthesis, these tin oxide nanoparticles were deposited on patterned copper substrate via thermal evaporator. Analysis for sensing ammonia solution was carried out using a three electrode system via electrochemical workstation. Electrochemical technique incorporates the results of cyclic voltammetry. The results confirmed that the SnO2-deposited copper (Cu) platform successfully detect the presence of ammonia solution in double-distilled water. These results were further analyzed and compared with each indium tin oxide (ITO)-coated glass and pure copper substrate. Moreover, the tin oxide-deposited copper platform showed better performance than ITO substrate.








Similar content being viewed by others
References
I.S. Helgadottir, P.P. Arquilliere, P. Brea, C.C. Santini, P.H. Haumesser, K. Richter, A.V. Mudring, M. Aouine, Microelectron. Eng. 107, 229–232 (2013)
S. Arya, P.K. Lehana, S.B. Rana, J. Electron. Mater. 46, 4604–4611 (2017)
C. Branci, N.B. Enjelloun, J. Sarradin, M.R. Ribes, Solid State Ion. 135, 169–174 (2000)
W.Q. Wu, D. Chen, Y.B. Cheng, R.A. Caruso, RRL Solar 1, 1700117 (2017)
G. Gaggiotti, A. Galdikas, S. Kaciulis, G. Mattogno, A. Setkus, J. Appl. Phys. 76, 4467 (1994)
R. Valaski, E. Silveira, L. Micaroni, I.A. Hummelgen, J. Solid State Electrochem. 5, 261–264 (2001)
J.F. McAleer, P.T. Moseley, J.O.W. Norris, D.E. Williams, P. Taylor, B.C. Tofield, Mater. Chem. Phys. 17, 577–583 (1987)
J.P. Ge, J. Sang, H.X. Zhang, X. Wang, Q. Peng, Y.D. Li, Sens. Actuators B Chem. 113, 937–943 (2006)
R. Adnan, N.A. Razana, I.A. Rahman, M.A. Farrukh, J. Chin. Chem. Soc. 57, 222–2292 (2010)
J. Zhu, B. Tay, Y. Ma, J. Mater. Lett. 60, 1003–1010 (2006)
S. Gnanam, V. Rajendran, J. Sol Gel Sci. Technol. 53, 555–559 (2010)
S. Phadungdhitidhada, P. Ruankham, A. Gardchareon, D. Wongratanaphisan, S. Choopun, Adv. Nat. Sci. Nanosci. Nanotechnol 8, 035004 (2017)
M.A.M. Akhir, K. Mohamed, H.L. Lee, S.A. Rezan, Proc. Chem. 19, 993–998 (2016)
S. Tazikeh, A. Akbari, A. Talebi, E. Talebi, Mater. Sci. Pol. 32, 98–101 (2014)
L.C. Nehru, C. Sanjeeviraja, J. Adv. Ceram. 3, 171–176 (2014)
S. Kim, H. Lee, C.M. Park, Y. Jung, J. Nanosci. Nanotechnol. 12, 1616–1619 (2014)
S.A. Papargyri, D.N. Tsipas, D.A. Papargyris, A.I. Botis, A.D. Papargyris, Solid State Phenom. 106, 57–62 (2005)
H. Deng, F.J. Lamelas, J.M. Hossenlopp, Chem. Mater. 15, 2429–2436 (2003)
A.V. Borhade, D.R. Tope, S.G. Gite, Arab. J. Chem. 10, S559–S567 (2017)
L. Jiang, G. Sun, Z. Zhou, S. Sun, Q. Wang, S. Yan, H. Li, J. Tian, J. Guo, B. Zhou, Q. Xin, J. Phys. Chem. B 109, 8774–8778 (2005)
G.W. Watt, J. Chem. Educ. 11, 339 (1934)
S.T. Dubas, V. Pimpan, Talanta 76, 29–33 (2008)
S. Pandey, G.K. Goswami, K.K. Nanda, Int. J. Biol. Macromol. 51, 583–589 (2012)
A.M. Giovannozzi, F. Pennecchi, P. Muller, P.B. Tivola, S. Roncari, A.M. Rossi, Anal. Bioanal. Chem. 407, 8423–8431 (2015)
G.R. Lima, L. Mota, A. Miklos, J. Angster, Z. Dubovski, M.G. da Silva, M. Sthel, H. Vargas, Appl. Phys. B. 117, 333–341 (2014)
W. Laminack, C. Baker, J.L. Gole, Air Qual. Atmos. Health 9, 231–239 (2016)
F. Valentini, V. Biagiotti, C. Lete. G. Palleschi, J. Wang, Sens. Actuators B Chem. 128, 326–333 (2007)
H.K. Boo, T.S. Ma, Microchim. Acta 66, 515–523 (1976)
M. Aziz, S.S. Abbas, W.R.W. Baharom, Mater. Lett. 91, 31–34 (2013)
S. Arya, A. Sharma, B. Singh, M. Riyas, P. Bandhoria, M. Aatif, V. Gupta, Opt. Mater. 78, 115–119 (2018)
F. Gu, S.F. Wang, M.K. Lu, G.J. Zhou, D. Xu, D.R. Yuan, J. Phys. Chem. B 108, 8119–8123 (2004)
U. Nithiyanantham, A. Ramadoss, S. Kundu, Dalton Trans. 45, 3506–3521 (2016)
G. Hussain, D.S. Silvester, Electroanalysis 30, 75–83 (2018)
Acknowledgements
This work is supported by SERB-DST (File no. EEQ/2016/000119).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arya, S., Riyas, M., Sharma, A. et al. Electrochemical detection of ammonia solution using tin oxide nanoparticles synthesized via sol–gel route. Appl. Phys. A 124, 538 (2018). https://doi.org/10.1007/s00339-018-1968-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-1968-8