Abstract
Objectives
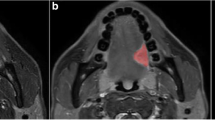
This study was conducted in order to evaluate a novel risk stratification model using dual-energy CT (DECT) texture analysis of head and neck squamous cell carcinoma (HNSCC) with machine learning to (1) predict associated cervical lymphadenopathy and (2) compare the accuracy of spectral versus single-energy (65 keV) texture evaluation for endpoint prediction.
Methods
Eighty-seven patients with HNSCC were evaluated. Texture feature extraction was performed on virtual monochromatic images (VMIs) at 65 keV alone or different sets of multi-energy VMIs ranging from 40 to 140 keV, in addition to iodine material decomposition maps and other clinical information. Random forests (RF) models were constructed for outcome prediction with internal cross-validation in addition to the use of separate randomly selected training (70%) and testing (30%) sets. Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined for predicting positive versus negative nodal status in the neck.
Results
Depending on the model used and subset of patients evaluated, an accuracy, sensitivity, specificity, PPV, and NPV of up to 88, 100, 67, 83, and 100%, respectively, could be achieved using multi-energy texture analysis. Texture evaluation of VMIs at 65 keV alone or in combination with only iodine maps had a much lower accuracy.
Conclusions
Multi-energy DECT texture analysis of HNSCC is superior to texture analysis of 65 keV VMIs and iodine maps alone and can be used to predict cervical nodal metastases with relatively high accuracy, providing information not currently available by expert evaluation of the primary tumor alone.
Key Points
• Texture features of HNSCC tumor are predictive of nodal status.
• Multi-energy texture analysis is superior to analysis of datasets at a single energy.
• Dual-energy CT texture analysis with machine learning can enhance noninvasive diagnostic tumor evaluation.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating curve
- CI:
-
Confidence intervals
- DECT:
-
Dual-energy CT
- HNSCC:
-
Head and neck squamous cell carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- RF:
-
Random forests
- ROI:
-
Region of interest
- VMI:
-
Virtual monochromatic image
References
Som PM, Brandwein-Gensler MS (2011) Lymph nodes of the neck. In: Som PM, Curtin HD (eds) Head and neck imaging. Mosby, St. Louis
Forghani R, Yu E, Levental M, Som PM, Curtin HD (2014) Imaging evaluation of lymphadenopathy and patterns of lymph node spread in head and neck cancer. Expert Rev Anticancer Ther. https://doi.org/10.1586/14737140.2015.978862:1-18
Kostakoglu L (2011) PET/CT Imaging. In: Som PM, Curtin HD (eds) Head and neck imaging. Mosby, St. Louis
D’Cruz AK, Vaish R, Kapre N et al (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373:521–529
Abu-Ghanem S, Yehuda M, Carmel NN et al (2016) Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 142:857–865
Paleri V, Urbano TG, Mehanna H et al (2016) Management of neck metastases in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 130:S161–S169
Liao LJ, Hsu WL, Wang CT, Lo WC, Lai MS (2016) Analysis of sentinel node biopsy combined with other diagnostic tools in staging cN0 head and neck cancer: a diagnostic meta-analysis. Head Neck 38:628–634
Medina JE (2017) Cancer of the neck. In: Myers J, Hanna E, Myers EN (eds) Cancer of the head and neck. Wolters Kluwer, Philadelphia, pp 427–453
Zhang H, Graham CM, Elci O et al (2013) Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 269:801–809
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJ (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087
Dang M, Lysack JT, Wu T et al (2015) MRI texture analysis predicts p53 status in head and neck squamous cell carcinoma. AJNR Am J Neuroradiol 36:166–170
Buch K, Fujita A, Li B, Kawashima Y, Qureshi MM, Sakai O (2015) Using texture analysis to determine human papillomavirus status of oropharyngeal squamous cell carcinomas on CT. AJNR Am J Neuroradiol 36:1343–1348
Leijenaar RT, Carvalho S, Hoebers FJ et al (2015) External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol 54:1423–1429
Liu J, Mao Y, Li Z et al (2016) Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J Magn Reson Imaging 44:445–455
Vallieres M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60:5471–5496
Bayanati H, Thornhill RE, Souza CA et al (2015) Quantitative CT texture and shape analysis: can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur Radiol 25:480–487
Andersen MB, Harders SW, Ganeshan B, Thygesen J, Torp Madsen HH, Rasmussen F (2016) CT texture analysis can help differentiate between malignant and benign lymph nodes in the mediastinum in patients suspected for lung cancer. Acta Radiol 57:669–676
Foncubierta-Rodriguez A, Jimenez del Toro OA, Platon A, Poletti PA, Muller H, Depeursinge A (2013) Benefits of texture analysis of dual energy CT for computer-aided pulmonary embolism detection. Conf Proc IEEE Eng Med Biol Soc 2013:3973–3976
Oldan J, He M, Wu T et al (2014) Pilot study: evaluation of dual-energy computed tomography measurement strategies for positron emission tomography correlation in pancreatic adenocarcinoma. J Digit Imaging 27:824–832
Depeursinge A, Foncubierta-Rodriguez A, Vargas A et al (2013) Rotation-covariant texture analysis of 4D dual-energy CT as an indicator of local pulmonary perfusion. 2013 IEEE 10th international symposium on biomedical imaging (ISBI), San Francisco, CA, USA, pp 145–148
Al Ajmi E, Forghani B, Reinhold C, Bayat M, Forghani R (2018) Spectral multi-energy CT texture analysis with machine learning for tissue classification: an investigation using classification of benign parotid tumours as a testing paradigm. Eur Radiol 28:2604–2611
Srinivasan A, Parker RA, Manjunathan A, Ibrahim M, Shah GV, Mukherji SK (2013) Differentiation of benign and malignant neck pathologies: preliminary experience using spectral computed tomography. J Comput Assist Tomogr 37:666–672
Lam S, Gupta R, Levental M, Yu E, Curtin HD, Forghani R (2015) Optimal virtual monochromatic images for evaluation of normal tissues and head and neck cancer using dual-energy CT. AJNR Am J Neuroradiol 36:1518–1524
Wichmann JL, Noske EM, Kraft J et al (2014) Virtual monoenergetic dual-energy computed tomography: optimization of kiloelectron volt settings in head and neck cancer. Invest Radiol 49:735–741
Forghani R, Levental M, Gupta R, Lam S, Dadfar N, Curtin HD (2015) Different spectral hounsfield unit curve and high-energy virtual monochromatic image characteristics of squamous cell carcinoma compared with nonossified thyroid cartilage. AJNR Am J Neuroradiol 36:1194–1200
Albrecht MH, Scholtz JE, Kraft J et al (2015) Assessment of an advanced monoenergetic reconstruction technique in dual-energy computed tomography of head and neck cancer. Eur Radiol 25:2493–2501
Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ (2014) Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol 24:574–580
Rizzo S, Radice D, Femia M et al (2018) Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol 28:760–769
Yang L, Luo D, Li L et al (2016) Differentiation of malignant cervical lymphadenopathy by dual-energy CT: a preliminary analysis. Sci Rep 6:31020
Yamauchi H, Buehler M, Goodsitt MM, Keshavarzi N, Srinivasan A (2016) Dual-energy CT-based differentiation of benign posttreatment changes from primary or recurrent malignancy of the head and neck: comparison of spectral Hounsfield units at 40 and 70 keV and iodine concentration. AJR Am J Roentgenol 206:580–587
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
Forghani R, Kelly H, Yu E et al (2017) Low-energy virtual monochromatic dual-energy computed tomography images for the evaluation of head and neck squamous cell carcinoma: a study of tumor visibility compared with single-energy computed tomography and user acceptance. J Comput Assist Tomogr 41:565–571
Forghani R (2015) Advanced dual-energy CT for head and neck cancer imaging. Expert Rev Anticancer Ther. https://doi.org/10.1586/14737140.2015.1108193:1-13
Lam S, Gupta R, Kelly H, Curtin HD, Forghani R (2015) Multiparametric evaluation of head and neck squamous cell carcinoma using a single-source dual-energy CT with fast kVp switching: state of the art. Cancers (Basel) 7:2201–2216
Forghani R, Srinivasan A, Forghani B (2017) Advanced tissue characterization and texture analysis using dual-energy computed tomography: horizons and emerging applications. Neuroimaging Clin N Am 27:533–546
Forghani R, De Man B, Gupta R (2017) Dual-energy computed tomography: physical principles, approaches to scanning, usage, and implementation: part 2. Neuroimaging Clin N Am 27:385–400
Kraft M, Ibrahim M, Spector M, Forghani R, Srinivasan A (2018) Comparison of virtual monochromatic series, iodine overlay maps, and single energy CT equivalent images in head and neck cancer conspicuity. Clin Imaging 48:26–31
Ueno Y, Forghani B, Forghani R et al (2017) Endometrial carcinoma: MR imaging-based texture model for preoperative risk stratification—a preliminary analysis. Radiology. https://doi.org/10.1148/radiol.2017161950:161950
Parikh J, Selmi M, Charles-Edwards G et al (2014) Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology 272:100–112
De Cecco CN, Ganeshan B, Ciolina M et al (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 50:239–245
Breiman L (2001) Random forests. Mach Learn 45:5–32
Caruana R, Karampatziakis N, Yessenalina A (2008) An empirical evaluation of supervised learning in high dimensions. ICML '08, Proceedings of the 25th International conference on Machine learning. ACM, Helsinki, pp 96–103
Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning: Data mining, inference, and prediction, Second edition. Springer Series in Statistics, Springer-Verlag
Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2:18–22
Yang X, Wu K, Li S et al (2017) MFAP5 and TNNC1: potential markers for predicting occult cervical lymphatic metastasis and prognosis in early stage tongue cancer. Oncotarget 8:2525–2535
Funding
This work was partly supported by a grant from the Rossy Cancer Network. R.F. is a clinical research scholar supported by the FRQS (Fonds de recherche en santé du Québec).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is R. Forghani.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: R.F. has acted as consultant and speaker for GE Healthcare and is a founding partner and stockholder of 4Intel Inc. B.F. is a founding partner and stockholder of 4Intel Inc.
Statistics and biometry
B.F. has significant statistical and informatics expertise and performed the mathematical and statistical analyses.
Informed consent
Ethics approval was obtained by the Institutional Review Board of the Jewish General Hospital (CIUSSS West-Central Montreal).
Ethical approval
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 48 kb)
Rights and permissions
About this article
Cite this article
Forghani, R., Chatterjee, A., Reinhold, C. et al. Head and neck squamous cell carcinoma: prediction of cervical lymph node metastasis by dual-energy CT texture analysis with machine learning. Eur Radiol 29, 6172–6181 (2019). https://doi.org/10.1007/s00330-019-06159-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06159-y