Abstract
Key message
Stress hormones, particularly jasmonic acid, influenced root growth, auxin levels, and transcription of auxin amidohydrolase BrIAR3 in Brassica rapa seedlings, while auxin conjugate synthetases BrGH3.1 and BrGH3.9 were down-regulated by all treatments.
Abstract
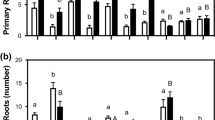
The influence of stress hormones: jasmonic acid (JA), salicylic acid (SA), and abscisic acid (ABA) on 1-day-old seedlings of Chinese cabbage (Brassica rapa L. ssp. pekinensis) was investigated with particular focus on auxin levels and the regulation of reversible auxin conjugation as a mechanism of auxin homeostasis. At the physiological level, stress hormones inhibited root growth, where JA was the most prominent inhibitor with an IC50 value 3.1 μM, which is one and two orders of magnitude lower than that found for ABA and SA, respectively. JA treatment significantly increased the total auxin content, by induction of free and conjugated forms. Also, the stress hormones affected the transcription of genes involved in the process of the reversible auxin conjugation: auxin amidohydrolases BrIAR3 and BrILL2, and auxin conjugate synthetases BrGH3.1 and BrGH3.9. JA treatment increased the transcript level of BrIAR3 two-fold, while it did not affect the transcription of BrILL2. SA and ABA down-regulated the transcription of both auxin amidohydrolase genes by 30 %. Transcription of both auxin conjugate synthetases was significantly down-regulated by all treatments by 30–70 %. Among the investigated biochemical stress markers, glutathione along with protein carbonylation appeared the most affected upon treatments. The redox status of the seedlings was shifted to the more oxidized state upon JA and ABA treatments, whereas SA caused more reduced redox state in comparison to the control. The principal component analysis visualized relationship among auxin and stress parameters upon treatments. Accordingly, the role of auxin in stress response of Brassica seedlings was discussed.






Similar content being viewed by others
References
Alam I, Lee DG, Kim KH, Park CH, Sharmin SA, Lee H, Oh KW, Yun BW, Lee BH (2010) Proteome analysis of soybean roots under waterlogging stress at an early vegetative stage. J Biosci 35:49–62
Bari R, Jones JDG (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 69:473–488
Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22:376–391
Belin C, Megies C, Hauserová E, Lopez-Molina L (2009) Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling. Plant Cell 21:2253–2268
Campanella JJ, Larko D, Smalley J (2003) A molecular phylogenomic analysis of the ILRI-like family of IAA amidohydrolase genes. Comp Funct Genomics 4:584–600
Campanella JJ, Smith SM, Leibu D, Wexler S, Ludwig-Müller J (2008) The auxin conjugate hydrolase family of Medicago truncatula and their expression during the interaction with two symbionts. J Plant Growth Regul 27:26–38
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 2. Academic Press, New York, pp 764–775
Chandler JW (2009) Auxin as compère in plant hormone crosstalk. Planta 231:1–12
Cohen JD, Baldi BG, Slovin JP (1986) 13C6-[benzene ring]-indole-3-acetic acid, a new internal standard for quantitative mass spectral analysis of indole-3-acetic acid in plants. Plant Physiol 80:14–19
Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B (1999) IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell 11:365–376
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 1(Web Server issue):W465–469
Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, Wang S (2008) Activation of the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 20:228–240
Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, Kazan K (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19:2225–2245
Domingo C, Andrés F, Tharreau D, Iglesias DJ, Talón M (2009) Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol Plant-Microbe Interac 22:201–210
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Griffith OW (1980) Detremination of glutathione and glutathione disulfide using glutathione reductase and 2-vinypyridine. Anal Biochem 106:207–212
Grsic S, Kirchheim B, Pieper K, Fritsch M, Hilgenberg W, Ludwig-Müller J (1999) Induction of auxin biosynthetic enzymes by jasmonic acid and in clubroot diseased Chinese cabbage plants. Physiol Plant 105:521–531
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z (2012) DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24:1815–1833
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Jung C, Lyou SH, Yeu SY, Kim MA, Rhee S, Kim M, Lee JS, Choi YD, Cheong JJ (2007) Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep 26:1053–1063
Junghans U, Polle A, Düchting P, Weiler E, Kuhlmann B, Gruber F, Teichmann T (2006) Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ 29:1519–1531
Khan S, Stone JM (2007) Arabidopsis thaliana GH3.9 influences primary root growth. Planta 226:21–34
Kinoshita N, Wang H, Kasahara H, Liu J, MacPherson C, Machida Y, Kamiya Y, Hannah MA, Chua NH (2012) IAA-Ala resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell 24:3590–3602
Koprivova A, Mugford ST, Kopriva S (2010) Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep 29:1157–1167
LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277:20446–20452
Ludwig-Müller J (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62:1757–1773
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K, Kamiya Y, Kasahara H (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108:18512–18517
Monzón GC, Pinedo M, Lamattina L, de la Canal L (2012) Sunflower root growth regulation: the role of jasmonic acid and its relation with auxins. Plant Growth Regul 66:129–136
Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM (2007) GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282:10036–10046
Pilet PE, Saugy M (1987) Effect on root growth of endogenous and applied IAA and ABA. Plant Physiol 83:33–38
Popko J, Hänsch R, Mendel RR, Polle A, Teichmann T (2010) The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol 12:242–258
Rahman A (2013) Auxin: a regulator of cold stress response. Physiol Plant 147:28–35
Rasmussen R (2001) Quantification on the light cycler. In: Meuer S, Wittwer C, Nakagawara K (eds) Rapid cycle real-time PCR, methods and applications. Springer Press,Heidelberg, pp 21–34
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate salicylate antagonism. Annu Rev Phytopathol 49:317–343
Savić B, Tomić S, Magnus V, Gruden K, Barle K, Grenković R, Ludwig-Müller J, Salopek-Sondi B (2009) Auxin amidohydrolases from Brassica rapa cleave the alanine conjugate of indolepropionic acid as a preferable substrate: a biochemical and modeling approach. Plant Cell Physiol 50:1587–1599
Schuller A, Ludwig-Müller J (2006) A family of auxin conjugate hydrolases from Brassica rapa: characterization and expression during clubroot disease. New Phytol 171:1–13
Shang J, Xi DH, Xu F, Wang SD, Cao S, Xu MY, Zhao PP, Wang JH, Jia SD, Zhang ZW, Yuan S, Lin HH (2011) A broad-spectrum, efficient and nontransgenic approach to control plant viruses by application of salicylic acid and jasmonic acid. Planta 233:299–308
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphor-molybdic-phosphotungstic acid reagents. Am J Enol Viticult 171:1–3
Srivastava MK, Dwivedi UN (1998) Salicylic acid modulates glutathione metabolism in pea seedlings. J Plant Physiol 153:409–414
Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89:6837–6840
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17:616–627
Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23:3961–3973
Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, Wu X, Cohen JD, Palme K, Li C (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511
Sun J, Chen Q, Qi L, Jiang H, Li S, Xu Y, Liu F, Zhou W, Pan J, Li X, Palme K, Li C (2011) Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol 191:360–375
Teichman T, Bolu-Arianto WH, Olbrich A, Langenfeld-Heyser R, Göbel C, Grzeganek P, Feussner I, Hänsch R, Polle A (2008) GH3:GUS reflects cell-specific developmental patterns and stress-induced changes in wood anatomy in the poplar stem. Tree Physiol 28:1305–1315
Titarenko E, Rojo E, León J, Sánchez-Serrano JJ (1997) Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol 115:817–826
Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, De Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, Genty B, Stubbs KA, Inze D, Van Breusegem F (2010) Perturbation of indole-3-butyric acid homeostasis by the UDP-Glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22:2660–2679
Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA dependent priming for callose. Plant J 38:119–130
Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17:1784–1790
Wang H, Tian C, Duan J, Wu K (2008) Research progresses on GH3s, one family of primary auxin-responsive genes. Plant Growth Regul 56:225–232
Wang L, Hua D, He J, Duan Y, Chen Z et al (2011) Auxin response factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet 7:e1002172. doi:10.1371/journal.pgen.1002172
Woldemariam MG, Onkokesung N, Baldwin IT, Galis I (2012) Jasmonoyl-L-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-L-isoleucine levels and attenuates plant defenses against herbivores. Plant J 72:758–767
Wu H, Wu X, Li Z, Duan L, Zhang M (2012) Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J Plant Growth Regul 31:113–123
Yuan S, Lin HH (2008) Role of salicylic acid in plant abiotic stress. Z Naturforsch 63:313–320
Zhang Z, Li Q, Li Z, Staswick PE, Wang M, Zhu Y, He Z (2007) Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol 145:450–464
Acknowledgments
This work was supported by the Croatian Ministry of Science, Education and Sports (Grant no. 098-0982913-2829), Deutscher Akademischer Austauschdienst (DAAD) German-Croatian mobility Grant no. 50751505, Alexander von Humboldt-Foundation (Institutional partnership), and a doctoral fellowship to Ana Smolko granted by the National Croatian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
A contribution to the Special Issue: Plant Hormone Signaling.
Rights and permissions
About this article
Cite this article
Salopek-Sondi, B., Šamec, D., Mihaljević, S. et al. Influence of stress hormones on the auxin homeostasis in Brassica rapa seedlings. Plant Cell Rep 32, 1031–1042 (2013). https://doi.org/10.1007/s00299-013-1412-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1412-7