Abstract
We describe the expression and immunogenicity of a recombinant chimeric protein (HAV VP1-Fc) consisting of human hepatitis A virus VP1 and an Fc antibody fragment using a replicating vector based on Beet curly top virus (BCTV) in Agrobacterium-infiltrated Nicotiana benthamiana leaves. Recombinant HAV VP1-Fc was expressed with a molecular mass of approximately 68 kDa. Recombinant HAV VP1-Fc, purified using Protein A Sepharose affinity chromatography, elicited production of specific IgG antibodies in the serum after intraperitoneal immunization. Following vaccination with recombinant HAV VP1-Fc protein, expressions of IFN-γ and IL-4 were increased in splenocytes at the time of sacrifice. Recombinant VP1-Fc from infiltrated tobacco plants can be used as an effective experimental immunogen for research into vaccine development.
Similar content being viewed by others
Introduction
Hepatitis A virus (HAV) is classified as a picornavirus with a single-stranded, positive-sense RNA genome encoding a single polyprotein that is subsequently processed into structural and nonstructural proteins. The structural proteins of HAV are divided into the polypeptides VP1, VP2, VP3, and VP4, which are the capsid polypeptides of the virus. VP1 appears to be the dominant structural protein among the capsid proteins. Studies of the reaction of HAV with monoclonal antibodies and the use of isolated structural proteins or synthetic peptides for induction of neutralizing antibodies all suggest that VP1 contains a major immunodominant epitope of HAV (Hughes et al. 1984; Stapleton and Lemon 1987; Hughes and Stanton 1985; Emini et al. 1985).
The use of recombinant subunit vaccines comprised antigenic peptides (epitopes) is a particularly attractive strategy for wide-scale immunization, when compared with formalin-inactivated HAV vaccines. Recombinant subunit vaccines offer potentially equivalent efficacy, but are much safer and, in some cases, easier to produce (Arnon 1991). Plants offer unique advantages for production of subunit vaccines in terms of scale, speed, costs, yield, and safety (Fisher et al. 2004).
Up to now, the majority of plant-derived antigenic peptides have been produced using transgenic plants (Fisher et al. 2004). However, transient expression is a viable alternative. A short production cycle coupled with optimization of expression yields large amounts of protein in a relatively short time period (Twyman et al. 2003). In this respect, we previously demonstrated the feasibility of a Beet curly top virus (BCTV)-based, transient expression vector system by expression of a high-level of recombinant green fluorescent protein (GFP) in Nicotiana benthamiana (Kim et al. 2007).
Production of large quantities of antigenic HAV VP1 would be necessary for immunological and biochemical studies directed toward vaccine development. Although recombinant VP1 has successfully been produced in Escherichia coli and insect cell systems (Ostermayr et al. 1987; Hamon et al. 1988; Lee et al. 2009), its expression in plants has not been investigated.
Therefore, in this study, HAV VP1 fused to the immunoglobin Fc fragment (HAV VP1-Fc) was expressed in tobacco leaves using a BCTV vector system. The fusion of heterologous peptides with Fc is known to provide stability and facilitate the purification process in a plant system (Obregon et al. 2006; Spitsin et al. 2009). We also performed an immunological assessment via intraperitoneal immunization of mice with the plant-derived vaccine antigen.
Materials and methods
DNA constructs
VP1 cDNA of hepatitis A virus (HAV) was amplified from pGEM-T/HAV-VP1 (Lee et al. 2009) via polymerase chain reaction (PCR) using sense (5′-GCGGCCGCAGTTGGAGATGATTCAG-3′) and antisense (5′-AGATCTCTCAAATCTTTTATCTTCCTC-3′) primers. The Fc fragment of human IgG cDNA (Gene Bank Accession No. AY172957) was amplified from total RNA extracted from human B cells IM-9 (Korean Cell Line Bank, Korea) using reverse transcription-PCR with oligonucleotide primers. The sense primer was 5′-AGATCTGCGGCCGCAGTTGAGCCCAAATCTTGTGAC-3′ and the antisense primer was 5′-AGATCTACCCGGGGACAGGGAGAGGC-3′. The amplified VP1 and human Fc sequences were then cloned into the pGEM-T vector (Promega, Madison, WI) to yield pGEM-T/VP1 and pGEM-T/Fc. The NotI–BglII fragment of pGEM-T/VP1 and the BglII fragment of pGEM-T/Fc were inserted between NotI and BglII of pImpactVector 1.3-tag (Plant Research International, Wageningen, The Netherlands), which includes the N-terminal signal peptide, C-terminal tags (c-myc and His6) and an ER retention signal (KDEL). The C-terminus of VP1 was fused to the Fc fragment of human IgG to yield pImpactVector 1.3-tag/VP1-Fc/c-myc-His6-KDEL. The VP1-Fc/c-myc-His6-KDEL sequences were amplified from pImpactVector 1.3-tag/VP1-Fc/c-myc-His6-KDEL via PCR using sense (5′-GATATCATGTCTCTTAGCCAGAAC-3′) and antisense (5′-ACTAGTTTAAAGTTCGTCCTTGTG -3′) primers and subcloned into the EcoRV and SpeI sites of pBCTVR, thereby yielding pCsVMV–BCTVR-VP1-Fc/c-myc-His6-KDEL (Fig. 1a). PCR was conducted using a Thermal Cycler (PE Biosystems, Foster City, CA) with a commercial PCR Mix (Takara, Japan) in a volume of 50 μl. All of the constructs were verified using restriction enzyme mapping and DNA sequencing.
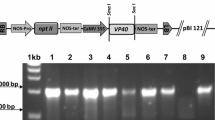
a Schematic diagram of the vector construct (pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL). Ovals mark the duplicated viral origin of replication, and REP indicates the viral gene block containing the viral L1 (REP), L2, L3 (REn), and L4 genes of Beet curly top virus (Kim et al. 2007). The region between the right (T R) and left (T L) borders also carries VP1-Fc (the sequence of the VP1 fused to the Fc fragment of human IgG), the CsVMV promoter from the Cassava vein mosaic virus, the nopaline synthase (NOS) transcription terminator, the signal peptide, C-terminal tag (c-myc-His6), the ER retention signal (KDEL), and the selection marker cassette (HPT) conferring resistance to hygromycin. T-DNA regions are not drawn to scale. b Replication of the pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL vector in Agrobacterium-infiltrated N. benthamiana leaves. A 10 μg of total DNA was separated on 0.9% agarose gel, transferred to a nylon membrane, and then hybridized with mixed 32P-labeled probes specific for VP1 and the λ/HindIII marker. Lane M contains λ/HindIII molecular weight markers. Lane N contains DNA sample isolated from non-inoculated N. benthamiana leaves. DNA isolated from leaves 5 days after infiltration with recombinant Agrobacterium containing pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL was either untreated (lane 1) or restricted with SalI (lane 2). c Western blot analysis of recombinant VP1-Fc in Agrobacterium-infiltrated leaves of N. benthamiana. A 50 μg of total proteins from extracts of infiltrated leaves was separated on 10% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated with goat antihuman IgG (Fc specific)-alkaline phosphatase conjugate. Lane M contains molecular weight markers. Lane N contains total protein isolated from non-inoculated N. benthamiana leaves. Lane C (negative control containing the vector backbone) contains total protein isolated from leaves 5 days after infiltration with recombinant Agrobacterium containing pCsVMV-BCTVR-c-myc-His6-KDEL. Lane 1 contains total protein isolated from leaves 5 days after infiltration with recombinant Agrobacterium containing pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL. The arrow indicates the recombinant VP1-Fc protein. d Quantification of the recombinant VP1-Fc expression level by a comparison with purified VP1-Fc standards. Western blot of extracts from Agrobacterium-infiltrated leaves of N. benthamiana is shown. A 10 μg of proteins from extracts of infiltrated leaves were separated on 10% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated with goat anti-human IgG (Fc specific)-peroxidase conjugate. Lane 1 contains total protein isolated from leaves 5 days after infiltration with recombinant Agrobacterium containing pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL. Lanes 2, 3 and 4 contain 30, 60 and 90 ng of purified VP1-Fc protein
Propagation of HAV
HAV strain HM175/18f was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and propagated in BS-C-1 cells (Korean Cell Line Bank) using T-75 culture flasks. BS-C-1 cells were maintained in RPMI-1640 medium (Thermo Scientific Hyclone, Logan, UT) containing 10% (v/v) fetal bovine serum (FBS; Thermo Scientific Hyclone) at 37°C in a humidified incubator with 5% CO2. Cultured BS-C-1 cells at 80% confluency were washed with phosphate buffered saline (PBS, pH 7.4), infected with HAV and incubated for 8 days in RPMI-1640 medium with 2% FBS. After two freeze–thaw cycles of cultures, the medium fraction containing HAV was separated by centrifugation at 10,000×g for 10 min.
Recombinant Agrobacterium tumefaciens strains and infiltration
The recombinant plant expression vector pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL was introduced into Agrobacterium tumefaciens GV3101 using electroporation, as described by Ainsworth et al. (1996). Recombinant A. tumefaciens GV3101 strains harboring pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL were infiltrated into the abaxial air spaces of 2–4-week-old Nicotiana benthamiana plants (Kim et al. 2007). Western blot analysis was performed at 5 days post-infiltration.
Western blot analysis
Proteins were extracted from infiltrated leaves (Ainsworth et al. 1996) followed by electrophoresis on 10% SDS–polyacrylamide gel. The fractionated proteins were transferred to nitrocellulose membrane (Hybond-C extra; Amersharm Pharmacia Biotech, Pittsburgh, PA). For alkaline phosphatase detection, membranes were blocked with 5% nonfat dried milk in TBST (TBS with 0.1% Tween 20) and then probed with goat anti-human IgG (Fc specific)-alkaline phosphatase conjugate (diluted to 1:10,000; Sigma, St. Louis, MO) for 1 h. After washing, a BCIP/NBT solution (Amresco, Solon, OH) was added and the reaction was quenched with distilled water. For ECL detection membranes were blocked with 5% nonfat dried milk in TBST and then probed with goat anti-human IgG (Fc specific)-peroxidase conjugate (diluted to 1:10,000; Sigma) for 1 h. After washing, protein bands were detected using an ECL Western blot detection kit (GE Healthcare, Sweden).
Purification of recombinant VP1-Fc
All steps were performed at 4°C. Recombinant VP1-Fc was purified using affinity fractionation with Protein A Sepharose 4 Fast Flow (GE Healthcare) according to the manufacturer’s recommendations. Protein extracts were dialyzed with binding buffer (20 mM sodium phosphate buffer, pH 7.0) and applied to a Protein A Sepharose 4 Fast Flow column. Weakly bound contaminating proteins were washed from the beads using binding buffer. Recombinant VP1-Fc was then eluted using 0.1 M glycine (pH 3.0), neutralized with 1 M Tris–HCl (pH 9.0), and dialyzed in PBS. For a control, recombinant VP1-Fc was purified using Ni–NTA resin (Qiagen, Valencia, CA) in a chromatography column (Novagen, Madison, WI) as described elsewhere (Lee et al. 2007). Protein concentrations were determined using a Bradford Protein Assay Kit (Bio-Rad, Hercules, CA) with BSA as a standard.
Deglycosylation of recombinant VP1-Fc
Purified recombinant VP1-Fc was digested using N-glycosidase (PNGase) F and O-glycosidase (Roche, Basel, Switzerland). PNGase F can cleave all types of N-glycans bound to aspargaine residues of proteins, except glycans containing α(1,3) fucose attached to the innermost GlcNAc. O-glycosidase releases the Gal β(1–3) GalNAc unit from O-glycans, which is bound to either serine or threonine as a core unit. For reactions containing a single enzyme, 50 ng of purified recombinant VP1-Fc was deglycosylated for 18 h at 37°C with either 10 mU of PNGase F in 50 mM sodium phosphate buffer (pH 7.5) or 15 μU of O-glycosidase in 50 mM sodium acetate buffer (pH 5.0). For reaction containing both enzymes, 50 ng of purified recombinant VP1-Fc was treated with a cocktail of 10 mU of PNGase F and 15 μU of O-glycosidase in 50 mM sodium phosphate buffer (pH 7.5) for 18 h at 37°C. Reactions were stopped by addition of SDS sample buffer. Samples were subjected to SDS-PAGE and Western blotting, as described previously.
Enzyme-linked immunosorbent assay (ELISA) of recombinant VP1-Fc proteins in Agrobacterium-infiltrated leaves
For quantification of recombinant VP1-Fc proteins, the general sandwich enzyme-linked immunosorbent assay (ELISA) protocol was used. The concentration of recombinant VP1-Fc protein in the protein extract of Agrobacterium-infiltrated N. benthamiana leaves was estimated by comparison to known amounts of purified recombinant VP1-Fc protein. A 96-well ELISA plate was coated with mouse anti-human IgG (Fc fragment specific) (200 ng/well; Jackson ImmunoResearch, West Grove, PA) in PBS overnight at room temperature (RT). The plate was washed three times with PBS containing 0.05% Tween-20 (PBST) and blocked using 1% BSA in PBS. After washing three times with PBST, diluted protein extracts and purified VP1-Fc samples were added to the wells, and the plate was incubated for 1 h at RT. After three washes, goat anti-human IgG (Fc specific)-peroxidase conjugate (diluted to 1:10,000) was used for detection. Finally, the plate was washed and developed for 30 min with TMB (3,3′,5,5′-tetramethylbenzidine) in phosphate-citrate buffer (pH 5.0) containing 0.002% hydrogen peroxide. The reaction was then stopped by the addition of 50 μl of 2 M H2SO4 to each well. The absorbance was determined at 450 nm using an ELISA reader (Bio-Tek Inc., Winooski, VT).
Animal experiments
Five-week-old female BALB/c mice were divided into two groups (5 mice per group) and immunized intraperitoneally with PBS and VP1-Fc purified from tobacco leaves. Mice were injected with 30 μg of VP1-Fc (or PBS as a control) with Freund’s adjuvant (FA) 4 times at 2-week intervals. Freund’s complete adjuvant (FCA) was used in the first immunization and Freund’s incomplete adjuvant (FIA) was used for subsequent booster injections. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Kyung Hee University.
Detection of antibodies
To determine specific antibodies produced in serum after intraperitoneal immunization, blood was collected from the retro-orbital plexus at 1 week after the third and fourth immunization (days 42 and 56), and each serum sample was separated using centrifugation at 10,000×g and stored at −70°C until use. Anti-VP1-Fc IgG antibodies in serum were assayed using ELISA. Briefly, a 96-well ELISA plate was coated overnight at 4°C with 100 μl of purified VP1-Fc (2 μg/ml) per well in a coating buffer (0.05 M carbonate–bicarbonate buffer, pH 9.0). The plate was washed three times with 200 μl of PBST. Serum diluted in PBST was added to each well and the plate was incubated for 1 h at RT. After washing with PBST, 100 μl of anti-mouse IgG-peroxidase conjugate (diluted in PBST to 1:30,000; Sigma) was added per well and incubated for 1 h at RT. The rest of the assay procedure, following washing and developing with TMB, was the same as described for enzyme-linked immunosorbent assay (ELISA) of recombinant VP1-Fc proteins in Agrobacterium-infiltrated leaves.
Splenocyte culture and cytokine detection
The procedure for isolating cells from the spleen was carried out as described previously (Lee et al. 2009). Briefly, spleens were surgically removed and gently crushed using the plunger of a disposable syringe on a Falcon cell strainer (BD Bioscience, Franklin Lakes, NJ). Red blood cells from the spleen were removed using ACK buffer [0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM ethylenediaminetetraacetic acid (EDTA) at pH 7.3]. After washing three times in RPMI-1640 medium, cells were resuspended in RPMI-1640 medium containing 10% FBS, 2-mercaptoethanol, and antibiotics, and were seeded at a density of 5 × 106 cells/ml onto a 24-well plate (Nunc, Denmark) for cytokine assay. After 48 or 72 h in the presence of the appropriate antigen, cell culture supernatants were harvested and applied to a sandwich ELISA system (BD Bioscience) for detection of IFN-γ and IL-4, according to the manufacturer’s instruction.
Cross-reactivity of anti-HAV VP1-Fc antibodies to native HAV antigen and HAV particles
Cross-reactivity of the antibodies raised after intraperitoneal immunization with VP1-Fc in BALB/c mice against HAV antigens (Meridian Life Science, Memphis, TN) was investigated. A 96-well ELISA plate was coated with 100 μl of hepatitis A viral antigen (1 μg/ml) per well in a coating buffer (0.05 M carbonate–bicarbonate buffer, pH 9.0) and left overnight at 4°C. The plate was washed three times with 200 μl of PBST. Sera were then diluted from 1:400 to 1:12,800 in PBST, added to each well, and incubated for 1 h at RT. After washing with PBST, the plate was incubated with 100 μl of anti-mouse IgG-peroxidase conjugate (diluted in PBST to 1:5,000) per well for 1 h at RT. The plate was washed and color developed according to the procedures described above. Cross-reactivity of the antibodies raised after intraperitoneal immunization with VP1-Fc in BALB/c mice against HAV (ATCC) particles was also investigated using Western blot analysis. The medium fraction of HAV-infected BS-C-1 cells was resolved on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dried milk in TBST and then incubated with PBS- or VP1-Fc-immunized serum (diluted to 1:100), followed by incubation with anti-mouse IgG-peroxidase conjugate (diluted to 1:10,000). The band was detected using the ECL Western blot detection kit (GE Healthcare).
Statistical analysis
All data are presented as mean ± SE. Student’s t test was used to compare different data groups (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Expression and purification of recombinant VP1-Fc in Agrobacterium-infiltrated leaves
Nicotiana benthamiana leaves were infiltrated with recombinant Agrobacterium tumefaciens GV3101 harboring pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL and harvested 5 days after infiltration. The pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL vector system includes two BCTV replication origins flanking the intact viral Rep and REn genes and VP1-Fc cDNA under control of the strong and constitutive CsVMV promoter (Fig. 1a). Southern hybridization analysis using a VP1-Fc-specific probe indicated that in Agrobacterium-infiltrated leaves receiving pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL, a smaller DNA species containing VP1-Fc and corresponding in size to sequences between the duplicated replication origins (i.e. replicating BCTV vector) was present at 5 days after infiltration (Fig. 1b).
Western blot analysis was performed using extracts prepared from leaves 5 days after infiltration with either pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL or negative control containing vector alone (Fig. 1c). Recombinant VP1-Fc with a molecular mass of approximately 68 kDa was present in leaves infiltrated with pCsVMV-BCTVR-VP1-Fc/c-myc-His6-KDEL, but not in non-inoculated leaves (Fig. 1c, lane N) or in leaves infiltrated with the negative control (Fig. 1c, lane C). The observed molecular weight is greater than predicted molecular weight (63 kDa) for the recombinant protein. The difference between these molecular weights could be a consequence of glycosylation. The expression level of VP1-Fc containing the endoplasmic reticulum (ER) retention signal in Agrobacterium-infiltrated leaves was estimated to be approximately 0.6% total soluble protein from tobacco leaf material when compared with purified VP1-Fc standards on a Western blot (Fig. 1d).
The VP1-Fc chimeric protein (approximately 68 kDa in size) was highly expressed in Agrobacterium-infiltrated leaves. Recombinant VP1-Fc was rapidly purified to near homogeneity using a protein A Sepharose affinity purification procedure. The purity of the protein was analyzed using SDS-PAGE and silver staining (Fig. 2a). Western blot analysis using an anti-human IgG (Fc specific)-alkaline phosphatase conjugate further confirmed the identity of the purified protein (Fig. 2b). No contaminating proteins were visible on silver nitrate-stained SDS-PAGE gel (Fig. 2a, lane 2). We obtained approximately 40% recovery of recombinant purified VP1-Fc via purification using Protein A Sepharose 4 Fast Flow from tobacco leaf material. Using non-reducing conditions, recombinant HAV VP1-Fc migrated at a size consistent with its assembly into dimers (Fig. 2c).
SDS-PAGE (a) and Western blot analysis (b) of recombinant VP1-Fc purified from tobacco leaves. M indicates molecular weight markers. Lane 1 total protein extract prior to affinity purification (1 μg), lane 2 pooled eluate containing 50 ng of protein after affinity purification. The arrows indicate the recombinant VP1-Fc. c Dimerization of recombinant VP1-Fc. Recombinant VP1-Fc protein purified under reducing (lane 1) or non-reducing (lane 2) conditions was resolved on 8% SDS-polyacrylamide gels and analyzed using Western blot analysis. d Deglycosylation of recombinant VP1-Fc. Purified recombinant VP1-Fc protein was treated with PNGase F (lane 2), O-glycosidase (lane 3), or with a cocktail of PNGase F and O-glycosidase (lane 4). M and 1 indicate molecular weight marker and non-treated purified recombinant VP1-Fc, respectively
As mentioned earlier, the molecular weight of recombinant VP1-Fc was greater than predicted which could be a consequence of glycosylation. Purified recombinant VP1-Fc was, therefore, treated with glycosidases and re-analyzed. As can be seen (Fig. 2d), digestion of recombinant VP1-Fc with PNGase F and O-glycoxidase resulted in a single band with a reduced molecular weight of approximately 63 kDa. This is consistent with the increased apparent molecular weight of purified recombinant VP1-Fc being due to glycosylation.
Immunogenicity of recombinant VP1-Fc in mice
BALB/c mice were injected with either VP1-Fc or PBS to determine the immunogenicity of plant-derived VP1. An enzyme-linked immunosorbent assay (ELISA) analysis revealed a strong serum IgG response to VP1-Fc in the immunized group (Fig. 3a). This indicates that recombinant VP1-Fc, when administered intraperitoneally with FA, has a potent immunogenicity and induces production of IgG class anti-VP1-Fc antibodies in mouse serum.
a Serum antibody response to VP1-Fc in BALB/c mice. BALB/c mice were immunized with 30 μg of purified VP1-Fc (or PBS as a control) and FA. After 7 days of the third immunization, specific IgG antibodies in antisera were measured using ELISA. The results from triplicate assays for all 5 mice per group are presented as mean ± SE. PBS (filled circle), VP1-Fc (open circle). b, c The concentrations of IFN-γ and IL-4 in splenocyte cell culture supernatants. Splenocytes from mice were stimulated with purified VP1-Fc antigen (or PBS as a control). After 48 or 72 h, supernatants were collected to examine the levels of IFN-γ (b) and IL-4 (c) using commercially available mice cytokine ELISA kits. Data are shown as mean ± SE of triplicate assays with splenocytes pooled from all five mice per group. PBS (filled square), VP1-Fc (open square)
Splenocytes prepared from immunized mice were then cultured in the presence of each antigen and synthesis of IFN-γ and IL-4 examined. As shown in Fig. 3b, splenocytes from mice immunized with antigen (VP1-Fc) and cultured in the presence of the antigen secreted large amounts of IFN-γ. This result indicates that VP1-Fc promotes formation of Th1-type cytokines. When splenocytes from mice immunized with VP1-Fc were cultured with VP1-Fc, production of IL-4 was increased approximately threefold more than when cultured in the absence of antigen (Fig. 3c). This indicates that VP1-Fc also promotes formation of Th2-type cytokines.
Differences probably exist in the physical properties of the recombinant and viral proteins. Therefore, we investigated the cross-reactivity of antibodies developed in intraperitoneally immunized BALB/c mice against HAV antigens (Meridian Life Science). Two serum groups were used for non-competitive indirect ELISA analysis. One group was from mice immunized with recombinant VP1-Fc and FA, while the other group (as a control) was from mice immunized with PBS and FA. When serum samples at a 1:400 dilution were applied to wells coated with HAV antigens, the mean ELISA values were 0.97 and 0.23, respectively, for the two groups (Fig. 4a).
Cross-reactivity of antibodies raised after intraperitoneal immunization of recombinant VP1-Fc in BALB/c mice against hepatitis A viral antigens. a ELISA titers of sera from BALB/c mice immunized with VP1-Fc antigen. The results from triplicate assays for all five mice per group are presented as mean ± SE. PBS (filled circle), VP1-Fc (open circle). b Western blot analysis of HAV particles using sera from BALB/c mice immunized with VP1-Fc antigen. The left panel shows the Western blot analysis using PBS-immunized serum. The right panel shows the Western blot analysis using VP1-Fc-immunized serum. 1 and 2 indicate HAV-free and HAV-infected BS-C-1 medium fractions, respectively. The arrow indicates the location of a band that reacted with VP1-Fc-immunized sera against HAV particles
The antibodies developed in intraperitoneally immunized BALB/c mice against HAV antigens were also tested for cross-reactivity with HAV (ATCC) particles. The two serum groups described above were used for Western blot analysis. As shown in Fig. 4b, a 33 kDa band in lane 2 of the right panel corresponds to a band for the VP1 protein of HAV particles. Thus, antisera from mice immunized with recombinant VP1-Fc and FA was reactive with only the HAV-infected BS-C-1 medium fraction. Taken together, our results indicate that antibodies produced by mice against plant-derived recombinant VP1-Fc most likely recognize epitopes in the target viral proteins.
Discussion
We used a BCTV-based, transient expression vector system to express recombinant HAV VP1-Fc in Agrobacterium-infiltrated N. benthamiana leaves. Fusion of the Fc fragment of the antibody and the ER retention signal KDEL to the C-terminus of VP1 facilitated detection and purification of antigens. When compared with purified VP1-Fc standards on a Western blot (Fig. 1d) the expression level of VP1-Fc was determined to be approximately 0.6% total soluble protein from tobacco leaf material, which is similar to the level estimated by ELISA. However, this level is lower than the level (1.5%) of GFP expression we reported elsewhere (Kim et al. 2007). Lower expression levels of human papillomavirus major CP L1 using a vector based on Bean yellow dwarf virus, when compared with EGFP have also been reported (Regnard et al. 2010).
The Fc antibody fragment of the chimeric VP1-Fc allowed the efficiency of purification to be improved using the protein A-based method, when compared with the Ni–NTA resin-based method. We initially attempted to purify VP1-Fc using the Ni–NTA resin-based method; however, the yield was low (<10% recovery from fresh leaves). We believe that the Fc-tag can be used as an alternative to the His-tag for single-step purification of recombinant proteins from other plant expression systems. Using non-reducing conditions, we have confirmed that recombinant HAV VP1-Fc is assembled into dimers. The ability of these molecules to dimerize might confer some structural advantage in terms of the recombinant protein stability expressed in tobacco leaves. The assembly status of a chimeric peptide has been suggested to be a determinant of stability for a recombinant HIV-1 p24-immunoglobulin fusion molecule (Obregon et al. 2006). The analysis of the chimeric VP1-Fc peptide sequence using the NetNGlyc 3.1 server (http://www.cbs.dk/services) and the OGPET 1.0 server (http://www.ogpet.utep.edu/OGPET) indicates that the chimeric VP1-Fc protein has two potential sites (N278, N429) for N-linked glycosylation, but does not contain any consensus sites for O-linked glycosylation. The results shown in Fig. 2d confirm that recombinant VP1-Fc does not contain any O-linked glycans, which can be removed by O-glycosidase.
We also examined the immunogenicity of the VP1-Fc derived from plant leaves in animal experiments. Plant-derived recombinant VP1-Fc produced specific IgG antibodies in serum after intraperitoneal immunization of mice. Levels of IgG antibodies specific for the plant-derived VP1-Fc were significantly increased after the third injection, indicating the effective stimulatory effect of VP1-Fc on B cell differentiation and maturation in mice. To our knowledge, this is the first demonstration that plant-derived VP1 is capable of inducing a strong IgG antibody response in mice after intraperitoneal immunization.
When considering the increase in IFN-γ expression, our results indicate a potential Th1 response due to plant-derived VP1-Fc in mice. The Th1 response can lead to a cytotoxic T cell response and virus clearance. IFN-γ plays an important role in the VP1-mediated T cell response since elevation of the IFN-γ level is considered to be related to Th1-type immunity. Our results also show that VP1-Fc induces a typical Th2 response in mice, which can eventually lead to a B cell response and production of specific antibodies. Hence, the cytokine profiles of spleen cells indicate that plant-derived VP1-Fc has a potent effect, inducing both cellular and humoral immunity. This could be an extremely useful feature for a plant-derived vaccine candidate targeted against HAV.
It is well known that generation of Th2 cells depends on IL-4. The mutually antagonistic effects of IL-4 and IFN-γ can regulate the Th1/Th2 balance and subsequent polarization (Rengarajan et al. 2000). For example, exposure of naïve helper T cells to IL-4 causes differentiation into Th2 cells at the beginning of an immune response. In addition, under a threshold level of IL-4, Th2 development is greatly favored over Th1, even if IFN-γ and IL-12 are present (Seder and Paul 1994). Our limited analysis of cytokine profiles from spleen cells implies that the recombinant VP1-Fc protein derived from plant leaves can be an ideal candidate as an effective vaccine for control of HAV infection. We observed a significant increase in the secretion of both IFN-γ (Th1-like) and IL-4 (Th2-like) with the recombinant HAV VP1-Fc protein, when compared with PBS, indicating co-stimulation of both Th1 and Th2 immune responses.
Although cell-mediated immunity is known to be responsible for controlling intracellular infections, such as HAV infection, humoral immunity is essential for controlling extracellular infections. Therefore, co-stimulation of both cellular and humoral immunity has been proposed as necessary for a host to control infections (Bertoletti and Gehring 2006). In this study, we did not determine the IgG isotypes in the serum, but immunization with the recombinant VP1-Fc did induce a greater amount of the specific IgG antibodies, compared to PBS, indicating strong induction of the humoral immune response against the recombinant VP1-Fc. Also, both non-competitive indirect ELISA and immunoblot analyses confirmed the specificity of immunized mice-derived serum response against HAV antigens and HAV particles, indicating that antibodies produced by mice against plant-derived recombinant VP1-Fc probably recognized epitopes in the target viral proteins.
We have shown that the BCTV-based expression system can be useful for expression of recombinant vaccine antigens, such as HAV VP1-Fc, in tobacco leaves. Our findings also highlight the potential of transient expression in plants for production of vaccine antigens. Transient expression through Agrobacterium-mediated infiltration is now considered a protein expression strategy that can potentially yield large amounts of protein. In addition, the short production cycle can reduce costs, as compared to the equivalent transgenic approach (Twyman et al. 2003).
It has been reported that fusion of the antigen with the IgG Fc fragment may increase the immunogenicity of the antigen (Obregon et al. 2006). However, Spitsin et al. (2009) reported no detectable fusion-related enhancement of immunogenicity or quality of the immune response for fusion of the avian flu antigen with the IgG Fc fragment. Our group also reported that the recombinant chimeric protein of a cancer antigen and the Fc fragment did not show any Fc effect in the antibody response against the antigen (Seok et al. 2010). Although further study is necessary to investigate the effect of Fc on the immune response in mice, it appears that Fc effects are unlikely, given our animal studies.
In summary, we report a strategy for the production of safe and inexpensive VP1 vaccine antigens against HAV. In this study, VP1 fused to the immunoglobin Fc fragment (VP1-Fc) was expressed in tobacco leaves using a BCTV replicating vector system. The high-yield potential of the BCTV replicating vector system greatly enhances the feasibility of production of plant-derived vaccine in tobacco leaves. It appears that fusion of heterologous peptides with the human immunoglobin Fc fragment facilitates the purification process in different expression systems. The ability of fusion molecules containing the Fc fragment to assemble together probably confers some structural advantage for protein stability. In addition, the glycosylated and highly charged Fc fragment enhances the solubility of hydrophobic proteins (Lo et al. 1998). Plant-derived recombinant chimeric proteins of the HAV capsid protein VP1 and the Fc antibody fragment elicited production of specific IgG in the serum after intraperitoneal immunization. Our findings show that functional expression of the major immunogen VP1-Fc using a replicating vector system based on Beet curly top virus in plant tissue provides a convenient source of recombinant VP1-Fc for research into vaccine development.
References
Ainsworth C, Benyon J, Buchanan-Wollaston V (1996) Techniques in plant molecular biology. Wye College Publications, London
Arnon R (1991) Synthetic peptides as the basis for vaccine design. Mol Immunol 28:209–215
Bertoletti A, Gehring AJ (2006) The immune response during hepatitis B virus infection. J Gen Virol 87:1439–1449
Emini EA, Hughes JV, Perlow DS, Boger J (1985) Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol 55:836–839
Fisher R, Stoger E, Schillberg S, Christou P, Twyman RM (2004) Plant-based production of biopharmaceuticals. Curr Opin Plant Biol 7:152–158
Hamon SA, Johnston JM, Ziegelhoffer T, Richards OC, Summers DF, Ehrenfeld E (1988) Expression of hepatitis A virus capsid sequences in insect cells. Virus Res 10:273–280
Hughes JV, Stanton LW (1985) Isolation and immunizations with hepatitis A viral structural proteins: induction of antiprotein, antiviral, and neutralizing responses. J Virol 55:395–401
Hughes JV, Stanton LW, Tomassini JE, Long WJ, Scolnick EM (1984) Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol 52:465–473
Kim KI, Sunter G, Bisaro DM, Chung IS (2007) Improved expression of recombinant GFP using a replicating vector based on Beet curly top virus in leaf-disks and infiltrated Nicotiana benthamiana leaves. Plant Mol Biol 64:103–112
Lee JM, Hwang-Bo J, Sohn BH, Chung IS (2007) Functional expression of recombinant canstatin in stably transformed Drosophila melanogaster S2 cells. Protein Expr Purif 52:258–264
Lee JM, Lee HH, Hwang-Bo J, Shon DH, Kim W, Chung IS (2009) Expression and immunogenicity of recombinant polypeptide VP1 of human hepatitis A virus in stably transformed fruitfly (Drosophila melanogaster) Schneider 2 cells. Biotechnol Appl Biochem 53:101–109
Lo KM, Sudo Y, Chen J, Li Y, Lan Y, Kong SM, Chen L, An Q, Gillies SD (1998) High level expression and secretion of Fc-X fusion proteins in mammalian cells. Prot Eng 11:495–500
Obregon P, Chargelegue D, Drake PM, Prada A, Nuttall J, Frigerio L, Ma JK (2006) HIV-1 p24-immunoglobin fusion molecule: a new strategy for plant-based protein production. Plant Biotechnol J 4:195–207
Ostermayr R, Helm K, Gauss-muller V, Winnacker EL, Deinhardt F (1987) Expression of hepatitis A virus cDNA in E. coli: antigenic VP1 recombinant proteins. J Virol 61:3645–3647
Regnard GL, Halley-Stott RP, Tanzer FL, Hitzeroth II, Rybicki EP (2010) High level protein expression in plants though the use of a novel autonomously replicating geminivirus shuttle vector. Plant Biotechnol J 8:38–46
Rengarajan J, Szabo SJ, Glimcher LH (2000) Transcriptional regulation of Th1/Th2 polarization. Immunol Today 21:479–483
Seder RA, Paul WE (1994) Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol 12:635–673
Seok YJ, Kim KI, Yoo KH, Hwang-Bo J, Lee HH, Shon DH, Ko KS, Kang HS, Lee KJ, Oh DB, Joung YH, Chung IS (2010) Expression and immunogenicity of a recombinant chimeric protein of human colorectal cancer antigen GA733-2 and an Fc antibody fragment in stably transformed Drosophila melanogaster S2 cells. Appl Biochem Biotechnol 162:1435–1445
Spitsin S, Andrianov V, Pogrebnyak N, Smirnov Y, Borisjuk N, Portocarrero C, Veguilla V, Koprowski H, Golovkin M (2009) Immunological assessment of plant-derived avian flu H5/HA1 variants. Vaccine 27:1289–1292
Stapleton JT, Lemon SM (1987) Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol 61:491–498
Twyman RM, Stoger E, Schillberg S, Christou P, Fisher R (2003) Molecular farming in plants: host systems and expression technology. Trend Biotechnol 21:570–578
Acknowledgments
This work was supported by a grant from the Biogreen 21 Project (200901FHT020814288).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. R. Liu.
H.Y. Chung and H.H. Lee contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Chung, H.Y., Lee, H.H., Kim, K.I. et al. Expression of a recombinant chimeric protein of hepatitis A virus VP1-Fc using a replicating vector based on Beet curly top virus in tobacco leaves and its immunogenicity in mice. Plant Cell Rep 30, 1513–1521 (2011). https://doi.org/10.1007/s00299-011-1062-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1062-6