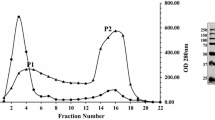
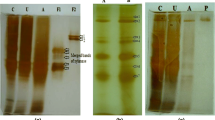
Abstract
Fourteen forms of endopolygalacturonases were purified from the culture medium of Sclerotinia sclerotiorum with ion exchange chromatographies. Individual forms differ in their isoelectric point but exhibit similar molecular masses. On the basis of the cross-reactions to antibodies raised against a purified acidic endopolygalacturonase, these forms could be divided into two related groups. Polygalacturonase activities constitute a complex enzyme system in which the extensive multiplicity is due to the expression of a large gene family.
Similar content being viewed by others
Literature Cited
Alghisi P, Favaron F (1995) Pectin-degrading enzymes and plant parasite interactions. Eur J Plant Pathol 101: 365–375
Bateman D, Basham H (1976) Degradation of plant cell walls and membranes by microbial enzymes. In: Heitefuss R, Williams P (eds) Physiological plant pathology. Berlin: Springer-Verlag, pp 316–355
Bussink H, Buxton F, Fraaye B, de Graaff L, Visser J (1992) The polygalacturonases of Aspergillus niger are encoded by a family of diverged genes. Eur J Biochem 208: 83–90
Byrde RJW (1979) Role of polysaccharide-degrading enzymes in microbial pathogenicity. In: Berkeley RCW, Gooday GW, Ellwood DC (eds) Microbial polysaccharides and polysaccharases. London: Academic Press, pp 417–436
Caprari C, Bergmann C, Micheli Q, Salvi C, Alberheim P, Darvill A, Cervone F, De Lorenzo G (1993) Fusarium moniliforme secretes four endopolygalacturonases derived from a single gene product. Physiol Mol Plant Pathol 43: 453–462
Favaron F, Alghisi P, Marciano P (1992) Characterization of two Sclerotinia sclerotiorum polygalacturonases with different abilities to elicit glyceollin in soybean. Plant Sci 83: 7–13
Fraissinet-Tachet L, Reymond-Cotton P, Fèvre M (1995) Characterization of a multigene family encoding an endopolygalacturonase in Sclerotinia sclerotiorum. Curr Genet 29: 96–100
Honda S, Nishimura Y, Takahashi M, Chiba H, Kakehi K (1982) A manual method for the spectrophotometric determination of reducing carbohydrates with 2-cyanoacetamide. Anal Biochem 119: 194–199
Keon JPR, Waksman G (1990) Common amino acid domain among endo polygalacturonases of Ascomycetes fungi. Appl Environ Microbiol 56: 2522–2528
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 222: 680–685
Marciano P, Di Lenna P, Magro P (1982) Polygalacturonase isoenzymes produced by Sclerotinia sclerotiorum in vivo and in vitro. Physiol Plant Pathol 20: 201–212
Reymond P, Deléage G, Rascle C, Fèvre M (1994) Cloning and sequence analysis of a polygalacturonase-encoding gene from the phytopathogenic fungus S. sclerotiorum. Gene 146: 233–237
Riou C, Freyssinet G, Fèvre M (1991a) Production of cell wall-degrading enzymes by the phytopathogenic fungus Sclerotinia sclerotiorum. Appl Environ Microbiol 57: 1478–1484
Riou C, Fraissinet-Tachet L, Freyssinet G, Fèvre M (1991b) Purification and characterization of extracellular pectinolytic enzymes produced by Sclerotinia sclerotiorum. Environ Microbiol 58: 578–583
Riou C, Fraissinet-Tachet L, Freyssinet G, Fèvre M (1992) Secretion of pectic isoenzymes by Sclerotinia sclerotiorum. FEMS Microbiol Lett 91: 231–238
Smith P, Krohn R, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D (1985) Measurement of protein using bincinchoninic acid. Anal Biochem 150: 76–85
Stratilova E, Markovic O, Strovinova D, Rexova-Benkova L, Jornvall H (1993) Aspergillus sp. polygalacturonase: multiplicity, divergence and structural patterns linking fungal, bacterial and plant polygalacturonases. J Prot Chem 12: 15–22
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Waksman G, Keon JP, Turner G (1991) Purification and characterization of two endopolygalacturonases from Sclerotinia sclerotiorum. Biochim Biophys Acta 1073: 43–48
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martel, MB., Létoublon, R. & Fěvre, M. Purification of endo polygalacturonases from Sclerotinia sclerotiorum: Multiplicity of the complex enzyme system. Current Microbiology 33, 243–248 (1996). https://doi.org/10.1007/s002849900107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002849900107