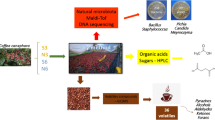
Abstract
The fermentation process of Yunnan arabica coffee is a typical wet fermentation. Its excellent quality is closely related to microbes in the process of fermentation. The purpose of this study was to isolate and identify the microorganisms in the wet method of coffee processing in Yunnan Province, China. Microbial community structure and dominant bacterial species were evaluated by traditional cultivated separation method and PCR-DGGE technology, and were further analyzed in combination with the changes of organic acid content, activity of pectinase, and physical parameters (pH and temperature). A large number of microorganisms which can produce pectinase were found. Among them, Enterobacter cowanii, Pantoea agglomerans, Enterobacteriaceae bacterium, and Rahnella aquatilis were the predominant gram-negative bacteria, Bacillus cereus was the predominant gram-positive bacterium, Pichia kluyveri, Hanseniaspora uvarum, and Pichia fermentans were the predominant yeasts, and all those are pectinase-producing microorganisms. As for the contents of organic acids, oxalic was the highest, followed by acetic and lactic acids. Butyrate and propionate, which were unfavorable during the fermentation period, were barely discovered.



Similar content being viewed by others
References
Avallone S, Guyot B, Brillouet JM, Olguin E, Guiraud JP (2001) Microbiological and biochemical study of coffee fermentation. Curr Microbiol 42:252–256
Avallone S, Brillouet JM, Guyot B, Olguin E, Guiraud JP (2002) Involvement of pectolytic micro-organisms in coffee fermentation. Int J Food Sci Tech 37:191–198
Barnett JA, Payne RW, Yarrow D (2000) Yeasts: characteristics and identification. In: Barnett JA, Payne RW, 3rd edn. Cambridge University Press, Cambridge, p 1139
Batista LR, Chalfoun SM, Prado G, Schwan RF, Wheals AE (2003) Toxigenic fungi associated with processed (green) coffee beans (Coffea Arabica L.). Int J Food Microniol 85:293–300
Batista LR, Chalfoun SM, Silva CF, Cirillo M, Varga EA, Prado G, Schwan RF (2009) Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control 20:784–790
Call HP, Walter J, Emies CC (1985) Maceration activity of an endopolygalacturonase from Candida macedoniensis. J Food Biochem 9:325–348
Coughlan MP, Mayer F (1991) The cellulose-decomposing bacteria and their enzymes systems. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes, vol 1. Springer, Berlin, pp 460–516
Cocolin L, Bisson LF, Mills DA (2000) Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol Lett 189:1069–1079
Danielle MV, Gilberto VP, Cristina FS, Luís RB, Rosane FS (2010) Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microbiol 27:1128–1135
Evangelista SR, Silva CF, Miguel MGPC, Cordeiro CS, Pinheiro ACM, Duarte WF, Schwan RF (2014) Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res Int 61:183–195
Evangelista SR, Miguel MGCP, Silva CF, Pinheiro ACM, Schwan RF (2015) Microbiological diversity associated with the spontaneous wet method of coffee fermentation. Int J Food Microbiol 210:102–112
Federici F (1985) Production, purification and partial characterisation of an endopolygalacturonase from Cryptococcus albidus var. albidus. Antonie Van Leeuwenhoek 51:139–150
Fowler MS, Leheup P, Cordier JL (1998) Cocoa, coffee and tea. In: Wood BJB (ed) Microbiology of femented foods, vol 1. Blackie Academic and Professional, London, pp 128–146
Gibson A, Butty M (1975) Overfermented coffee beans (“Stinkers”): a method for their detection and elimination. Association scientifique internationale du Café (edited by ASIC). In: 7th international scientific colloqium on coffee, Hamburg. Paris: Association Scientifique Internationale du Cafe (ASIC), pp 141–152
Kurtzman CP, Pichia EC (1998) Hansen emend. Kurtzman. In: Kurtzman CP, Fell JW (eds) The yeasts. A taxonomic study. Elsevier, Amsterdam, pp 273–352
Kohli P, Gupta R (2015) Alkaline pectinases: a review. Biocatal Agric Biotechnol 4:279–285
Kapoor M, Beg QK, Bhushan B, Singh K, Dadhich KS, Hoondal GS (2001) Application of an alkaline and thermostable polygalacturonase from Bacillus sp. MG-cp-2 in degumming of ramie (Boehmerianivea) and sunn hemp (Crotalaria juncea) bastfibers. Process Biochem 36:803–807
Licciardi R, Pereira RGFA, Mendonça LMVL, Furtad EF (2005) Avaliação físico-química de cafés torrados emoídos, de diferentes marcas comerciais, da Região Sul de Minas Gerais. Ciênc Tecnol Aliment 25:425–429
Lopez CI, Bautista E, Moreno E, Dentan E (1989) Factors related to the formation of over fermented coffee beans during the wet processing method and storage of coffee. In: Proceedings of 13th ASIC. Colombiap, pp 373–384
Lashermes P, Andrade AC, Etienne H (2008) Genomics of tropical crop plants. In: Moore PH, Ming R (eds) Genomics of coffee: one of the world’s largest traded commodities. Springer, New York, pp 203–225
Masoud W, Cesar LB, Jespersen L, Jakobsen M (2004) Yeast involved in fermentation of Coffea arabica in East Africa determined by genotyping and by direct denaturating gradient gel electrophoresis. Yeast 21:549–556
Masoud W, Jespersen L (2006) Pectin degrading enzymes in yeasts involved in fermentation of Coffee arabica in East Africa. Int J Food Microbiol 110:291–296
Masoud W, Leif P, Mogens J (2005) Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 22:1133–1142
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electroforesis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Payne C, Bruce A (2001) The yeast Debaryomyces hansenii as a short term biological control agent against fungal apoilage of sawn Pinus sylvestris timber. Biol Control 22:22–28
Parissa AT, Angélique GC, Francis D (2011) Yeasts: an attractive source of pectinase-from gene expression to potential applications: a review. Process Biochem 46:1525–1537
Phutela U, Vikram Dhuna, Sandhu S, Chadha BS (2005) Pectinase and polygalacturonase production by a thermophilic aspergillus fumigatus isolated from decomposting orange peels. J Microbiol 36:63–69
Poondla V, Bandikari R, Subramanyam R, Obulam VSR (2015) Low temperature active pectinases production by Saccharomyces cerevisiae isolate and their characterization. Biocatal Agric Biotechnol 4:70–76
Rehman HU, Aman A, Silipo A, Qader SAU, Molinaro A, Ansari A (2013) Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem 139:1081–1086
Sanchez J, Guiraud JP, Gazy P (1984) A study of the polygalacturonase activity of several yeast strains isolated from cocoa. Appl Microbiol Biotechnol 20:262–267
Silva CF, Batista LR, Abreu LM, Dias ES, Schwan RF (2008) Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol 25:951–957
Silva CF, Schwan RF, Dias ES, Wheals AE (2000) Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int J Food Microbiol 60:251–260
Smith MT (1998) Hanseniaspora Zikes. In: Kurtzman CP, Fell JW (eds) The yeasts. A taxonomic study. Elsevier, Amsterdam, pp 214–220
Stratilova E, Breierova E, Vadkertiova R, Malavikova A, Slavikova E (1998) The adaptability of the methylotrophic yeast Candida boidinii in media containing pectin substances. Can J Microbiol 44:116–120
Theuil PA, Claisse AG, Duchiron F (2011) Yeasts: an attractive source of pectinases—From gene expression to potential applications: a review. Process Biochem 46:1525–1537
Uzuner S, Cekmecelioglu D (2015) Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J Mol Catal B Enzym 113:62–67
Van PW, Castelein JM (1972) Study of the pectinolytic microflore, particularly the Enterobacteriaceae, from fermenting coffee in the Congo. J Food Sci 37:171–174
Vieira HD (2008) Plant parasitic nematodes of coffee. In: Souza M (ed) Coffee: the plant and its cultivation. Springer, Dordrecht, pp 3–18
Vaughn RH, Jaklibczyk T, Macmillanj D (1969) Some pink yeasts associated with softening of olives. Appl Environ Microbiol 18:771–775
Vilela DM, Pereira GVDM, Silva CF, Batista LR, Schwan RF (2010) Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microbiol 27:1128–1135
Wrigley G (1988) Coffee, chapter 2, 10, 11. Longman Scientific & Technical, London
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols. Academic Press, San Diego, p 317
Acknowledgments
This research is supported by Yunnan Provincial Science and Technology Department (No. 2012AB006).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, X., Dong, H., Yang, P. et al. Culture-Dependent and -Independent Methods to Investigate the Predominant Microorganisms Associated with Wet Processed Coffee. Curr Microbiol 73, 190–195 (2016). https://doi.org/10.1007/s00284-016-1047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1047-3