Abstract
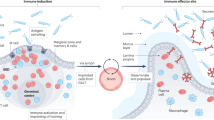
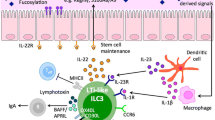
Mucosal lymphocyte homeostasis involves the dynamic interaction of enteric microbiota, the intestinal host epithelium, and the mucosal immune system. Dysregulation of mucosal lymphocyte homeostasis results in a variety of intestinal disorders, notably inflammatory bowel diseases like ulcerative colitis and Crohn’s disease. One key cellular component regulating homeostasis are B lymphocytes that reside in gut-associated lymphoid tissue. This compartment includes Peyer’s patches, isolated lymphoid follicles, lamina propria, and mesenteric lymph nodes. Recent data have pointed to two new and exciting aspects of B cells in the gut. First, there has been progress on identification and functional analysis of abundant isolated lymphoid follicle B cells that are key mediators of IgA genesis. Second, several groups have now clarified the functional identification and characterization of immunoregulatory B cells in the gut. This review examines the novel aspects of these B cells, and examines how each plays a role in mediating mucosal homeostasis in this bacteria-laden compartment.

Similar content being viewed by others
References
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 73:1131S
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 3:710
Lehrer RI, Ganz T (2002) Defensins of vertebrate animals. Curr Opin Immunol. 14:96
Ouellette AJ, Bevins CL (2001) Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis 7:43
Gewirtz AT, Neish AS, Madara JL (2002) Mechanisms of active intestinal inflammation and potential down-regulation via lipoxins. Adv Exp Med Biol. 507:229
Gewirtz AT, Liu Y, Sitaraman SV, Madara JL (2002) Intestinal epithelial pathobiology: past, present and future. Best Pract Res Clin Gastroenterol 16:851
Madara JL (1997) Review article: pathobiology of neutrophil interactions with intestinal epithelia. Aliment Pharmacol Ther 11 Suppl 3:57
Clark, MA, Jepson MA (2003) Intestinal M cells and their role in bacterial infection. Int J Med Microbiol 293:17
Kucharzik T, Lugering N, Rautenberg K, Lugering A, Schmidt MA, Stoll R, Domschke W (2000) Role of M cells in intestinal barrier function. Ann N Y Acad Sci 915:171
Neutra, MR, Mantis NJ, Kraehenbuhl JP (2001) Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol 2:1004
McCracken VJ, Lorenz RG (2001) The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol 3:1
Fagarasan S, Honjo T (2003) Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol 3:63
Lambolez F, Azogui O, Joret AM, Garcia C, Boehmer H von, Di Santo J, Ezine S, Rocha B (2002) Characterization of T cell differentiation in the murine gut. J Exp Med 195:437
Ishikawa H, Saito H, Suzuki K, Oida T, Kanamori Y (1999) New gut associated lymphoid tissue “cryptopatches” breed murine intestinal intraepithelial T cell precursors. Immunol Res 20:243
Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H (1998) Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280:275
Bendelac A, Bonneville M, Kearney JF (2001) Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol 1:177
Benlagha K, Park SH, Guinamard R, Forestier C, Karlsson L, Chang CH, Bendelac A (2004) Mechanisms governing B cell developmental defects in invariant chain-deficient mice. J Immunol 172:2076
Martin F, Kearney JF (2000) B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev 175:70
Moore MA (2004) Commentary: the role of cell migration in the ontogeny of the lymphoid system. Stem Cells Dev 13:1
Kunkel EJ, Butcher EC (2003) Plasma-cell homing. Nat Rev Immunol 3:822
MacDonald TT (2003) The mucosal immune system. Parasite Immunol 25:235
Nishikawa S, Honda K, Vieira P, Yoshida H (2003) Organogenesis of peripheral lymphoid organs. Immunol Rev 195:72
Mowat AM (2003) Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3:331
Moghaddami M, Cummins A, Mayrhofer G (1998) Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 115:1414
Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H (2002) Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 168:57
Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD (2003) Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol 170:5475
Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T (2002) Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298:1424
Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K (2004) B cell receptor signal strength determines B cell fate. Nat Immunol 5:317
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422:164
Lanning D, Zhu X, Zhai SK, Knight KL (2000) Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol Rev 175:214
Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM (1989) Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol 19:2335
Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H (2004) IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol 172:6259
Cariappa A, Pillai S (2002) Antigen-dependent B-cell development. Curr Opin Immunol 14:241
Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S (2001) The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity 14:603
Martin F, Kearney JF (2000) Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12:39
Dalwadi H, Wei B, Schrage M, Su TT, Rawlings DJ, Braun J (2003) B cell developmental requirement for the G alpha i2 gene. J Immunol 170:1707
Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT (2002) Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol 14:659
Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr (1996) Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 184:2271
Gonnella PA, Waldner HP, Weiner HL (2001) B cell-deficient (mu MT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol 166:4456
Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK (2002) Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velázquez, P., Wei, B. & Braun, J. Surveillance B lymphocytes and mucosal immunoregulation. Springer Semin Immun 26, 453–462 (2005). https://doi.org/10.1007/s00281-004-0189-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-004-0189-8