Abstract
Purpose
The artemisinin class of anti-malarial drugs has shown significant anti-cancer activity in pre-clinical models. Proposed anti-cancer mechanisms include DNA damage, inhibition of angiogenesis, TRAIL-mediated apoptosis, and inhibition of signaling pathways. We performed a phase I study to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of intravenous artesunate (IV AS).
Methods
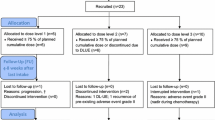
Patients were enrolled in an accelerated titration dose escalation study with planned dose levels of 8, 12, 18, 25, 34 and 45 mg/kg given on days 1 and 8 of a 21-day cycle. Toxicities were assessed using the NCI CTCAE (ver. 4.0), and response was assessed using RECIST criteria (version 1.1). Pharmacokinetic (PK) studies were performed during cycle 1.
Results
A total of 19 pts were enrolled, 18 of whom were evaluable for toxicity and 15 were evaluable for efficacy. DLTs were seen at dosages of 12 (1 of 6 patients), 18 (1 of 6) and 25 mg/kg (2 of 2), and were neutropenic fever (Gr 4), hypersensitivity reaction (Gr 3), liver function test abnormalities (Gr 3/4) along with neutropenic fever, and nausea/vomiting (Gr 3) despite supportive care. The MTD was determined to be 18 mg/kg. No responses were observed, while four patients had stable disease, including three with prolonged stable disease for 8, 10, and 11 cycles, for a disease control rate of 27%. PK parameters of AS and its active metabolite, dihydroartemisinin (DHA), correlated with dose.
Conclusion
The MTD of intravenous artesunate is 18 mg/kg on this schedule. Treatment was well tolerated. Modest clinical activity was seen in this pre-treated population.
ClinicalTrials.gov Identifier
NCT02353026.


Similar content being viewed by others
References
Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y (1993) Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob Agents Chemother 37(5):1108–1114
Lin AJ, Ager AL Jr, Klayman DL (1994) The antimalarial activity of dihydroartemisinin derivatives by transdermal application. Am J Trop Med Hyg 50(6):777–783
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91:41–46
Moore Lai H, Li JR, Ren RL, McDougall JA, Singh NP, Choud CK (1995) Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett 98:83–87
Nakase I, Lai H, Singh NP, Sasaki T (2008) Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm 354:28–33
Hou J, Wang D, Zhang R, Wang H (2008) Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14(17):5519–5530
Efferth T, Briehl MM, Tome ME (2003) Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol 4:1231–1235
Chen HH, Zhou HJ, Fang X (2003) Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res 48(3):231–236
Efferth T, Ramirez T, Gebhart E, Halatsch ME (2004) Combination treatment of glioblastoma multiforme cell lines with the anti-malarial artesunate and the epidermal growth factor receptor tyrosine kinase inhibitor OSI-774. Biochem Pharmacol 67(9):1689–1700
Rinner B, Siegl V, Pürstner P, Efferth T, Brem B, Greger H, Pfragner R (2004) Activity of novel plant extracts against medullary thyroid carcinoma cells. Anticancer Res 24(2A):495–500
Dell’Eva R, Pfeffer U, Vené R, Anfosso L, Forlani A, Albini A, Efferth T (2004) Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol 68:2359–2366
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ, Cha IH (2007) Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck 29(4):335–340
Zhou HJ, Wang WQ, Wu GD, Lee J, Li A (2007) Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol 47(2–3):131–138
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J, Fan SJ (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 28(7):1045–1056
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2:e693
Youns M, Efferth T, Reichling J, Fellenberg K, Bauer A, Hoheisel JD (2009) Gene expression profiling identifies novel key players involved in the cytotoxic effect of Artesunate on pancreatic cancer cells. Biochem Pharmacol 78(3):273–283
Li S, Xue F, Cheng Z, Yang X, Wang S, Geng F, Pan L (2009) Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFkappaB p65. Int J Hematol 90(4):513–521
Du JH, Zhang HD, Ma ZJ, Ji KM (2010) Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother Pharmacol 65(5):895–902
Michaelis M, Kleinschmidt MC, Barth S, Rothweiler F, Geiler J, Breitling R, Mayer B, Deubzer H, Witt O, Kreuter J, Doerr HW, Cinatl J, Cinatl J Jr (2010) Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem Pharmacol 79(2):130–136
Rasheed SA, Efferth T, Asangani IA, Allgayer H (2010) First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer 127(6):1475–1485
Sertel S, Eichhorn T, Simon CH, Plinkert PK, Johnson SW, Efferth T (2010) Pharmacogenomic identification of c-Myc/Max-regulated genes associated with cytotoxicity of artesunate towards human colon, ovarian and lung cancer cell lines. Molecules 15(4):2886–2910
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286(8):6587–6601
Thanaketpaisarn O, Waiwut P, Sakurai H, Saiki I (2011) Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-κB and PI3K/Akt signaling pathways. Int J Oncol 39(1):279–285
Singh NP, Verma KB (2002) Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch Oncol 10:279–280
Berger TG, Dieckmann D, Efferth T, Schultz ES, Funk JO, Baur A, Schuler G (2005) Artesunate in the treatment of metastatic uveal melanoma—first experiences. Oncol Rep 14(6):1599 – 603
Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O’Neill PM, Park BK (2007) Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem 282:9372–9382
Li PC, Lam E, Roos WP, Zdzienicka MZ, Kaina B, Efferth T (2008) Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res 68(11):4347–4351
Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK (2011) The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem 286(2):987–996
Disbrow GL, Baege AC, Kierpiec KA, Yuan H, Centeno JA, Thibodeaux CA, Hartmann D, Schlegel R (2005) Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65(23):10854–10861
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, Ross DD, Funk JO (2003) Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 64:382–394
Wang L, Chen T, Qu J, Wei X (2009) Quantitative analysis of caspase-3 activation by fitting fluorescence emission spectra in living cells. Micron 40(8):811–820
Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX (2007) Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int J Cancer 121(6):1360–1365
Konkimalla VB, McCubrey JA, Efferth T (2009) The role of downstream signaling pathways of the epidermal growth factor receptor for Artesunate’s activity in cancer cells. Curr Cancer Drug Targ 9(1):72–80
Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X, Xue D (2010) Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol 136(6):897–903
Chen HH, Zhou HJ, Wang WQ, Wu GD (2004) Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol 53:423–432
Anfosso L, Efferth T, Albini A, Pfeffer U (2006) Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenom J 6(4):269–278
Sieber S, Gdynia G, Roth W, Bonavida B, Efferth T (2009) Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int J Oncol 35(1):149–158
Bhaw-Luximon A, Jhurry D (2017) Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives. Cancer Chemother Pharmacol 79(3):451–466
Hien TT, White NJ (1993) Qinghaosu Lancet 341:603–608
U.S. Army Medical Material Development Activity (2012) Intravenous Artesunate for Severe Malaria (IND No. 64,769), Investigator Brochure
Dondorp A, Nosten F, Stepniewska K, Day N, White N (2005) Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366:717–725
Newton PN, Angus BJ, Chierakul W, Dondorp A, Ruangveerayuth R, Silamut K, Teerapong P, Suputtamongkol Y, Looareesuwan S, White NJ (2003) Randomized comparison of artesunate and quinine in the treatment of severe falciparum malaria. Clin Infect Dis 37:7–16
Leonardi E, Gilvary G, White NJ, Nosten F (2001) Severe allergic reactions to oral artesunate: a report of two cases. Trans R Soc Trop Med Hyg 95:182–183
Barradell LB, Fitton A (1995) Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs 50(4):714–741
Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, Thu le TA, Hung NC, Pirmohamed M, Park BK, Edwards G (2002) Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos 30(9):1005–1012
Acknowledgements
We thank the patients and their families who participated in this trial. We thank Colonel Bryan Smith, at US Army Medical Materiel Development Activity (USAMMDA), who led the development of intravenous artesunate for the US Army. We thank Dr. Merrill Egorin for assistance in the design of the dosing regimen used in this study. Finally, we thank Dr. Robert Robey for assistance in aspects of data interpretation.
Funding
The project was partially supported by the Ruesch Center for the Cure of Gastrointestinal Cancers and Award Number P30CA051008 from the National Cancer Institute. Further support came from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, the United States Army, or the United States Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author John Deeken declares he has no conflict of interest. Author Hongkun Wang declares she has no conflict of interest. Author Marion Hartley declares she has no conflict of interest. Author Amrita K. Cheema declares she has no conflict of interest. Author Brandon Smaglo declares he has no conflict of interest. Author Jimmy J. Hwang declares he has no conflict of interest. Author Aiwu Ruth He declares she has no conflict of interest. Author Louis M. Weiner declares he has no conflict of interest. Author John L. Marshall declares he has no conflict of interest. Author Giuseppe Giaccone declares he has no conflict of interest. Author Stephen Liu declares he has no conflict of interest. Author Jim Luecht declares he has no conflict of interest. Author Jay Y. Spiegel declares he has no conflict of interest. Author Michael J. Pishvaian declares he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Deeken, J.F., Wang, H., Hartley, M. et al. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol 81, 587–596 (2018). https://doi.org/10.1007/s00280-018-3533-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3533-8