Abstract
Purpose
Endostar is a recombinant human endostatin with antiangiogenic properties that has been useful in treating a wide range of cancers and shows promise for use in combination treatment for advanced colorectal cancer. This study aimed to evaluate the drug safety and tolerability of continuous intravenous infusion (CIV) of endostar in combination with modified FOLFOX6 (mFOLFOX6) as an initial therapy in advanced colorectal cancer patients.
Methods
This was a single-center, single-arm, open, dose-escalation study in patients with advanced colorectal cancer at Fudan University Shanghai Cancer Center between August 2010 and January 2012. A total of 21 patients were included. Standard dosage of mFOLFOX6 was used. CIV endostar commenced on day 4 to day 14 ascending from 7.5 to 15, 30, 45, 60, and 75 mg/m2/day. Primary outcomes were dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) of CIV endostar in combination with mFOLFOX6. Secondary outcomes were pharmacokinetic parameters. Physical examination, performance status, standard blood tests and electrocardiograms were performed.
Results
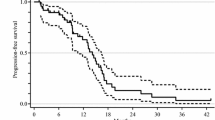
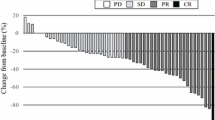
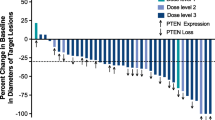
MTD was 75 mg/m2/day. Adverse events included leucopenia (n = 17, 81 %), neutropenia (n = 12, 57.1 %), anemia (n = 5, 23.8 %), anorexia (n = 6, 28.6 %) and constipation (n = 4, 19.0 %). One patient with an allergic reaction stopped chemotherapy. Two patients stopped endostar treatment, one with level 3 ventricular premature beat (DLT at 15 mg/m2/day) and one with a level 1 ventricular arrhythmia (30 mg/m2/day). The main ECG changes were ST-segment and T wave changes. Exposure to endostar and CIV dose was linear between 7.5 and 30 mg/m2/day (R 2 = 0.974).
Conclusions
Endostar in combination with mFOLFOX6 was generally safe and well tolerated.

Similar content being viewed by others
References
Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J (2014) Annual report on status of cancer in China, 2010. Chin J Cancer Res 26(1):48–58. doi:10.3978/j.issn.1000-9604.2014.01.08
Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, Liedke PE, Pramesh CS, Badovinac-Crnjevic T, Sheikine Y, Chen Z, Qiao YL, Shao Z, Wu YL, Fan D, Chow LW, Wang J, Zhang Q, Yu S, Shen G, He J, Purushotham A, Sullivan R, Badwe R, Banavali SD, Nair R, Kumar L, Parikh P, Subramanian S, Chaturvedi P, Iyer S, Shastri SS, Digumarti R, Soto-Perez-de-Celis E, Adilbay D, Semiglazov V, Orlov S, Kaidarova D, Tsimafeyeu I, Tatishchev S, Danishevskiy KD, Hurlbert M, Vail C, St. Louis J, Chan A (2014) Challenges to effective cancer control in China, India, and Russia. Lancet Oncol 15(5):489–538. doi:10.1016/S1470-2045(14)70029-4
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. doi:10.1002/ijc.25516
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet 383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
Benson AB, 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ, Fuchs CS, Grem JL, Hunt S, Kamel A, Leong LA, Lin E, May KS, Mulcahy MF, Murphy K, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Small W, Jr, Sofocleous CT, Venook AP, Willett CG, Gregory KM, Freedman-Cass DA (2013) Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 11(2):141–152; quiz 152
Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, Fakih MG, Fleshman J Jr, Fuchs C, Grem JL, Kiel K, Knol JA, Leong LA, Lin E, Mulcahy MF, Rao S, Ryan DP, Saltz L, Shibata D, Skibber JM, Sofocleous C, Thomas J, Venook AP, Willett C, National Comprehensive Cancer N (2009) NCCN clinical practice guidelines in oncology: rectal cancer. J Natl Compr Canc Netw 7(8):838–881
Arnold D, Seufferlein T (2010) Targeted treatments in colorectal cancer: state of the art and future perspectives. Gut 59(6):838–858. doi:10.1136/gut.2009.196006
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. doi:10.1200/JCO.2004.05.113
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. doi:10.1200/JCO.2007.14.9930
Nishina T, Takano Y, Denda T, Yasui H, Takeda K, Ura T, Esaki T, Okuyama Y, Kondo K, Takahashi Y, Sugiyama Y, Muro K (2013) A phase II clinical study of mFOLFOX6 plus bevacizumab as first-line therapy for Japanese advanced/recurrent colorectal cancer patients. Jpn J Clin Oncol 43(11):1080–1086. doi:10.1093/jjco/hyt127
Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, Catalano V, Ficarelli R, Maltese P, Bisonni R, Masi G, Schiavon G, Giordani P, Giustini L, Falcone A, Tonini G, Silva R, Mattioli R, Floriani I, Magnani M (2007) Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol 25(10):1247–1254. doi:10.1200/JCO.2006.08.1844
Chen YC, Tzeng CH, Chen PM, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS, Wang WS (2010) Influence of GSTP1 I105 V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci 101(2):530–535. doi:10.1111/j.1349-7006.2009.01418.x
Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26(21):3523–3529. doi:10.1200/JCO.2007.15.4138
Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D, First Bi (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 20(11):1842–1847. doi:10.1093/annonc/mdp233
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd, Eastern Cooperative Oncology Group Study E (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544. doi:10.1200/JCO.2006.09.6305
Macedo LT, da Costa Lima AB, Sasse AD (2012) Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer 12:89. doi:10.1186/1471-2407-12-89
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2):277–285
Folkman J (2006) Antiangiogenesis in cancer therapy–endostatin and its mechanisms of action. Exp Cell Res 312(5):594–607. doi:10.1016/j.yexcr.2005.11.015
Huang X, Wong MK, Zhao Q, Zhu Z, Wang KZ, Huang N, Ye C, Gorelik E, Li M (2001) Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res 61(2):478–481
Han B, Xiu Q, Wang H, Shen J, Gu A, Luo Y, Bai C, Guo S, Liu W, Zhuang Z, Zhang Y, Zhao Y, Jiang L, Zhou J, Jin X (2011) A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel–carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncol 6(6):1104–1109. doi:10.1097/JTO.0b013e3182166b6b
Sun L, Ye HY, Zhang YH, Guan YS, Wu H (2007) Epidermal growth factor receptor antibody plus recombinant human endostatin in treatment of hepatic metastases after remnant gastric cancer resection. World J Gastroenterol 13(45):6115–6118
Xu HX, Huang XE, Qian ZY, Xu X, Li Y, Li CG (2011) Clinical observation of Endostar(R) combined with chemotherapy in advanced colorectal cancer patients. Asian Pac J Cancer Prev 12(11):3087–3090
Zhou JF, Bai CM, Wang YZ, Li XY, Cheng YJ, Chen SC (2011) Endostar combined with chemotherapy for treatment of metastatic colorectal and gastric cancer: a pilot study. Chin Med J (Engl) 124(24):4299–4303
Jin F, Ji H, Jia C, Brockmeier U, Hermann DM, Metzen E, Zhu Y, Chi B (2012) Synergistic antitumor effects of endostar in combination with oxaliplatin via inhibition of HIF and CXCR4 in the colorectal cell line SW1116. PLoS ONE 7(10):e47161. doi:10.1371/journal.pone.0047161
Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116(10):2610–2621
Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Schultz L, Mikkelsen T (2010) Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol 99(2):237–242. doi:10.1007/s11060-010-0121-0
Kisker O, Becker CM, Prox D, Fannon M, D’Amato R, Flynn E, Fogler WE, Sim BK, Allred EN, Pirie-Shepherd SR, Folkman J (2001) Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res 61(20):7669–7674
Leong S, Eckhardt SG, Chan E, Messersmith WA, Spratlin J, Camidge DR, Diab S, Khosravan R, Lin X, Chow Maneval E, Lockhart AC (2012) A phase I study of sunitinib combined with modified FOLFOX6 in patients with advanced solid tumors. Cancer Chemother Pharmacol 70(1):65–74. doi:10.1007/s00280-012-1880-4
Yoshino T, Yamazaki K, Hamaguchi T, Shimada Y, Kato K, Yasui H, Boku N, Lechuga MJ, Hirohashi T, Shibata A, Hashigaki S, Li Y, Ohtsu A (2012) Phase I study of sunitinib plus modified FOLFOX6 in Japanese patients with treatment-naive colorectal cancer. Anticancer Res 32(3):973–979
Kato T, Muro K, Yamaguchi K, Bando H, Hazama S, Amagai K, Baba H, Denda T, Shi X, Fukase K, Skamoto J, Mishima H (2012) Cediranib in combination with mFOLFOX6 in Japanese patients with metastatic colorectal cancer: results from the randomised phase II part of a phase I/II study. Ann Oncol 23(4):933–941. doi:10.1093/annonc/mdr359
Sakamoto Y, Beppu T, Miyamoto Y, Okabe H, Ida S, Imai K, Chikamoto A, Watanabe M, Takamori H, Baba H (2013) Feasibility and short-term outcome of adjuvant FOLFOX after resection of colorectal liver metastases. J Hepatobiliary Pancreat Sci 20(3):307–312. doi:10.1007/s00534-012-0523-9
Tsutsumi S, Watanabe R, Tabe Y, Fujii T, Morita H, Kigure W, Kato T, Yamauchi H, Asao T, Kuwano H (2011) Scheduled prospective tri-weekly modified FOLFOX6 maintenance chemotherapy in the treatment of metastatic colorectal cancer. Hepatogastroenterology 58(112):1930–1932. doi:10.5754/hge10110
Conflict of interest
The authors have full control of all primary data and that we agree to allow the journal to review their data if requested. We declare that we have no conflict of interest.
Ethical standard
The study protocol was approved by the ethics committee of Fudan University Shanghai Cancer Center (100585-3) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Guo, W., Cao, J. et al. Endostar in combination with modified FOLFOX6 as an initial therapy in advanced colorectal cancer patients: a phase I clinical trial. Cancer Chemother Pharmacol 75, 547–557 (2015). https://doi.org/10.1007/s00280-014-2656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2656-9