Abstract
Purpose
Dinaciclib is a novel selective inhibitor of cyclin-dependent kinase (CDK)1, CDK2, CDK5, and CDK9. We conducted a phase I study to investigate the effects of dinaciclib when administered with rituximab.
Methods
In this phase I nonrandomized dose-escalation 3 + 3 trial, patients with relapsed/refractory chronic lymphocytic leukemia (CLL) were treated with dinaciclib and rituximab. Dinaciclib was administered intravenously (IV) over 2 h on days 1, 8 and 15 in cycles 2–13 (28-day cycles). Rituximab 375 mg/m2 was administered IV on days 1, 8, 15 and 22 in cycle 1 (28-day cycle) and on day 1 during cycle 3–13. Rituximab was not administered in cycle 2. Rituximab and dinaciclib were given alone in cycles 1 and 2, respectively, and in combination in cycles 3–13. Primary objectives included determination of the recommended phase II dose of dinaciclib and evaluation of pharmacokinetics (PK) when administered with rituximab.
Results
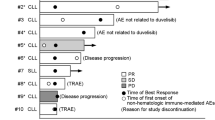
Five patients completed the study due to early termination. All presented with drug-related adverse events (AEs), but no dose-limiting toxicities were observed. The most commonly observed toxicities included hematological, digestive and metabolic AEs. However, no tumor lysis syndrome has been reported in the study. Four patients achieved stable disease, and one patient achieved complete response according to 2008 iwCLL criteria at cycle 3. PK samples were collected from 5 patients, and no obvious interaction between dinaciclib and rituximab was observed.
Conclusions
Limited data from this study shows dinaciclib in combination with rituximab was well tolerated and revealed encouraging clinical activity in relapsed/refractory CLL patients.

Similar content being viewed by others
References
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer M et al (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376:1164–1174
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al (2005) Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 23:4079–4088
Tam CS, O’Brien S, Weirda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 112:975–980
Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, Burger J, Koller C, Lerner S, Kantarjian H, Wierda WG (2011) Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 117:3016–3024
Cuthill K, Devereux S (2013) How I treat patients with relapsed chronic lymphocytic leukaemia. Br J Haematol 163:423–435
Cortelezzi AM, Sciume M, Liberati AM, Vincenti D, Cuneo A, Laurenti L et al (2014) Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia 28:642–648
Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, Sargent R, Burger JA, Farrajoli A (2013) Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 31:584–591
Rodriguez-Vicente AE, Diaz MG, Hernandez-Rivas JM (2013) Chronic lymphocytic leukemia: a clinical and molecular heterogenous disease. Cancer Genet 206:49–62
Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou YM, Statkevich P, Yao SL, Bannerji R (2013) A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 11:259
Parry D, Guzi T, Shanahan F, Davis N, Prabjavalkar D, Wiswell D et al (2010) Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther 9:2344–2353
Blachly JS, Byrd JC (2013) Emerging drug profile: cyclin-dependent kinase inhibitors. Leuk Lymphoma 54:2133–2143
Bose P, Simmons GL, Grant S (2013) Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs 22:723–738
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166
Morgan DO (1995) Principles of CDK regulation. Nature 374:131–134
Sausville EA (2002) Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med 8(4 Suppl):S32–S37
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24:1770–1783
Lim S, Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093
Gallorini M, Cataldi A, di Giacomo V (2012) Cyclin-dependent kinase modulators and cancer therapy. BioDrugs 26:377–391
Canavese M, Santo L, Raje N (2012) Cyclin dependent kinases in cancer: potential for therapeutic intervention. Cancer Biol Ther 13:451–457
Johnson AJ, Smith LL, Wagner AJ, Hessler J, Flynn JM, Jones JA, Zhang X et al (2010) Dinaciclib (SCH727965) is a novel cyclin dependent kinase inhibitor that promotes selective apoptosis in CLL cells and abrogates the protective effects of microenvironment cytokines. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 971
Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer J, Altiok S, Pledger WJ (2011) The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells. Mol Cancer Ther 10:1018–1027
Desai BM, Villanueva J, Nguyen TT, Lioni M, Xiao M, Kong J et al (2013) The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS ONE 8:e59588
Abdullah C, Wang X, Becker D (2011) Expression analysis and molecular targeting of cyclin-dependent kinases in advanced melanoma. Cell Cycle 10:977–988
Fu W, Sharma SS, Ma L, Chu B, Bui MM, Reed D, Pledger WJ (2013) Apoptosis of osteosarcoma cultures by the combination of the cyclin-dependent kinase inhibitor SCH727965 and a heat shock protein 90 inhibitor. Cell Death Dis 4:e566
Feldmann G, Mishra A, Bisht S, Karikari C, Garrido-Laguna I, Rasheed Z et al (2011) Cyclin-dependent kinase inhibitor dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther 12:598–609
Robak T (2012) Rituximab for chronic lymphocytic leukemia. Expert Opin Biol Ther 12:503–515
Lepretre S, Jager U, Janssens A, Leblond V, Nikitin E, Robak T, Wendtner CM (2012) The value of rituximab for the treatment of fludarabine-refractory chronic lymphocytic leukemia: a systematic review and qualitative analysis of the literature. Leuk Lymphoma 53:820–829
Griffin MM, Morley N (2013) Rituximab in the treatment of non-Hodgkin’s lymphoma—a critical evaluation of randomized controlled trials. Expert Opin Biol Ther 13:803–811
Weiner GJ (2010) Rituximab: mechanism of action. Semin Hematol 47:115–123
Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC (2002) The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 99:1038–1043
Hussain SR, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, Lucas DM, Byrd JC (2007) Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res 13:2144–2150
Burger K, Muhl B, Rohrmoser M, Coordes B, Heidemann M, Kellner M et al (2013) Cyclin-dependent kinase 9 links RNA polymerase II transcription to processing of ribosomal RNA. J Biol Chem 288:21173–21183
Flynn JM, Jones JA, Andritsos L, Blum KA, Johnson AJ, Hessler J, et al (2010) Update on the phase I study of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): confirmation of clinical activity and feasibility of long-term administration. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 1396
Flynn JM, Jones JA, Andritsos L, Blum KA, Johnson AJ, Hessler J, et al (2011) Phase I study of the CDK inhibitor dinaciclib (SCH 727965) in patients (pts) with relapsed/refractory CLL. Presented at the 2011 ASCO annual meeting, 3–7, June 2011, Chicago, IL, Abstract 6623
Baiocchi RA, Flynn JM, Jones JA, Blum KA, Hofmeister CC, Poon J, et al (2010) Early evidence of anti-lymphoma activity of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in heavily pre-treated low grade lymphoma and diffuse large cell lymphoma patients. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 3966
Flynn JM, Andritsos LA, Jones JA, Johnson AJ, Maddocks K, Wiley E, et al (2013) Dinaciclib (SCH 727965) is a novel cyclin-dependent kinase (CDK) inhibitor that exhibits activity in patients with relapsed or refractory chronic lymphocytic leukemia (CLL). Presented at the 55th ASH annual meeting and exposition, 7–10, December 2013, New Orleans, LA, Abstract 871
Sadowska M, Muvarak N, Lapidus RG, Sausville EA, Bannerji R, Gojo I (2010) Single agent activity of the cyclin-dependent kinase (CDK) inhibitor dinaciclib (SCH 727965) in acute myeloid and lymphoid leukemia cells. Presented at the 53rd ASH annual meeting and exposition, 10–13, December 2011, San Diego, CA, Abstract 3981
Mita MM, Mita AC, Moseley J, Poon J, Small KA, Jou Y, et al (2011) A phase I study of the CDK inhibitor dinaciclib (SCH 727965) administered every 3 weeks in patients (pts) with advanced malignancies: final results. Presented at the 2011 ASCO annual meeting, 3–7, June 2011, Chicago, IL, Abstract 3080
Acknowledgments
The authors would like to thank Dr. Jérôme Couturier (Institut Curie, Paris, France), Pr. Frédéric Davi (Hôpital Pitié-Salpétrière, Paris, France), Dr. Claire Mathiot (Institut Curie, Paris, France), Dr. Vincent Servois (Institut Curie, Paris, France), for their contributions to biological and radiological assessments of the study and Kristen Lewis of Merck & Co., Inc., Whitehouse Station, NJ for editorial assistance. The study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Whitehouse Station, NJ).
Conflict of interest
CF, MG, CE, MZ, MPS and CLT have no conflicts of interest to disclose. KS, EI, NS, DZ and HZ are current or former employees of Merck & Co., Inc., Whitehouse Station, NJ and may own stock/stock options in the company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabre, C., Gobbi, M., Ezzili, C. et al. Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol 74, 1057–1064 (2014). https://doi.org/10.1007/s00280-014-2583-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2583-9