Abstract
Purpose
Patupilone (EPO906) is a novel potent microtubule stabilizer, which has been evaluated for cancer treatment. A novel physiologically based pharmacokinetics (PBPK) model was developed based on nonclinical data to predict the disposition of patupilone in cancer patients.
Methods
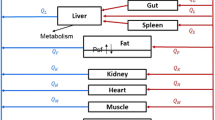
After a single intravenous dose (1.2 mg/kg) in male Han–Wistar rats, the tissue distribution of 14C-patupilone was investigated by quantitative whole-body autoradiography (QWBA). The blood radioactivity and patupilone concentration were determined by LC–MS/MS and liquid scintillation counting. A novel PBPK model was developed based on rat tissue concentration data to predict blood concentration–time profiles of patupilone in cancer patients. PBPK parameters derived from the rat were applied to a human PBPK model. Phase I clinical pharmacokinetic data in Caucasian and Japanese cancer patients at various doses ranging from 0.75 to 10 mg/m2 were successfully described using the PBPK approach.
Results
Patupilone dispositions in lung, heart, muscle, spleen, liver, brain, adipose, and testes of rats were well described using the PBPK model developed assuming a perfusion rate-limited distribution between different compartments. For skin and bone marrow, concentration–time profiles were modeled assuming a permeability-limited distribution between different compartments. The simulated human pharmacokinetic profiles from the PBPK model showed good agreement with observed clinical pharmacokinetic data, where the model predicted AUC, t 1/2, V ss, and CL values were within approximately twofold of the observed values for all dose groups.
Conclusions
The distribution of patupilone in rats was well described by a PBPK model based on measured tissue distribution profiles generated by QWBA combined with metabolism data. The human PBPK model adequately predicted blood pharmacokinetics of patupilone in cancer patients. The PBPK model based upon preclinical tissue distribution data can aid in successful prediction of pharmacokinetics in humans.






Similar content being viewed by others
References
Altmann KH, Wartmann M, O’Reilly T (2000) Epothilones and related structures—a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta 1470:M79–M91
Austin RP, Barton P, Cockroft SL, Wenlock MC, Riley RJ (2002) The influence of nonspecific microsomal binding on apparent intrinsic clearance, and its prediction from physicochemical properties. Drug Metab Dispos 30:1497–1503
Beardsley EK, Saad F, Eigl B, Venner P, Hotte S, Winquist E, Ko YJ, Sridhar SS, Chi KN (2009) A phase II study of patupilone in patients (pts) with metastatic castration-resistant prostate cancer (CRPC) who have progressed after docetaxel. J Clin Oncol 27:1–6
Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM (1995) Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 55:2325–2333
Brezniceanu LM, Deroussent A, Gu H, Mangold JB, Schiller H, Gross G, Cresteil T (2008) Oxidative metabolism of epothilones A and B (Patupilone) by cytochromes P450: involvement of CYP3A and CYP2C. Open Drug Metab J 2:14–23
Buey RM, Diaz JF, Andreu JM, O’Brate A, Giannakakou P, Nicolaou KC, Sasmal PK, Ritzen A, Namoto K (2004) Interaction of epothilone analogs with the paclitaxel binding site: Relationship between binding affinity, microtubule stabilization, and cytotoxicity. Chem Biol 11:225–236
Casado E, Tabernero J, Melichar B, Bridgewater J, Bennouna J, Delord J, Sopala M, Sklenar I, Cheung W, Johri A (2006) Patupilone in chemotherapy-pretreated patients with advanced colorectal cancer (CRC) receiving nutritional support and intensive diarrhea management: a phase I multicenter trial. J Clin Oncol. 2006 ASCO annual meeting proceedings part I, vol 24, no 18S (June 20 Supplement), 3593 [abstract]
Chi KN, Beardsley E, Eigl B, Venner P, Hotte SJ, Winquist E, Ko YJ, Saad F (2008) A phase 2 study of patupilone in patients with metastatic hormone refractory prostate cancer (Hrpc) who have progressed after docetaxel. J Clin Oncol. 2008 ASCO annual meetings, vol 26, (May 20 Supplement), 5166 [abstract]
Coe RA (2000) Quantitative whole-body autoradiography. Regul Toxicol Pharmacol 31:S1–S3
De Buck SS, Sinha VK, Fenu LA, Gilissen RA, Mackie CE, Nijsen MJ (2007) The prediction of drug metabolism, tissue distribution, and bioavailability of 50 structurally diverse compounds in rat using mechanism-based absorption, distribution, and metabolism prediction tools. Drug Metab Dispos 35:649–659
De Buck SS, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RAHJ (2007) Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos 35:1766–1780
Gore M, Forster M, Kaye S, Oza A, Sklenar I, Johri A, Cheung W, Zaknoen S (2007) A phase lb and pharmacokinetic trial of patupilone combined with carboplatin in patients with advanced cancer. Clin Cancer Res 13:4178–4184
Heimbach T, Lakshminarayana SB, Hu W, He H (2009) Practical anticipation of human efficacious doses and pharmacokinetics using in vitro and preclinical in vivo data. AAPS J 11:602–614
Jones HM, Gardner IB, Watson KJ (2009) Modelling and PBPK simulation in drug discovery. AAPS J 11:155–166
Jones HM, Parrott N, Jorga K, Lave T (2006) A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet 45:511–542
Jones RD, Jones HM, Rowland M, Gibson CR, Yates JW, Chien JY, Ring BJ, Adkison KK, Ku MS, He H, Vuppugalla R, Marathe P, Fischer V, Dutta S, Sinha VK, Bjornsson T, Lave T, Poulin P (2011) PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 2: comparative assessment of prediction methods of human volume of distribution. J Pharm Sci 100:4074–4084
Kilford PJ, Gertz M, Houston JB, Galetin A (2008) Hepatocellular binding of drugs: correction for unbound fraction in hepatocyte incubations using microsomal binding or drug lipophilicity data. Drug Metab Dispos 36:1194–1197
McSheehy R, O’Reilly MT, Wartmann M, Maira M, Allegrini P, Brueggen J (2007) Patupilone, the novel microtubule stabilizer (MTS), retains activity against human colon tumour cells over-expressing P-gp in vitro and in vivo; comparison with other MTS. EJC Suppl 14th European Cancer Conference (ECCO 14), vol 5, p 63, 323 [abstract]
Melichar B, Tabernero J, Casado E, Bridgewater J, Hamm J, Sklenar I, Holland J, Cheung W, Zaknoen S, Johri A (2005) Phase I dose optimization trial of patupilone in previously treated patients (pts) with advanced colon cancer (ACC). J Clin Oncol 23:292s
Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y (2001) Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos 29:1316–1324
O’Reilly T, McSheehy PMJ, Wenger F, Hattenberger M, Muller M, Vaxelaire J, Altmann KH, Wartmann M (2005) Patupilone (epothilone B, EPO906) inhibits growth and metastasis of experimental prostate tumors in vivo. Prostate 65:231–240
O’Reilly T, Wartmann M, Brueggen J, Allegrini PR, Floersheimer A, Maira M, McSheehy PMJ (2008) Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol 62:1045–1054
O’Reilly T, Wartmann M, Maira SM, Hattenberger M, Vaxelaire J, Muller M, Ferretti S, Buchdunger E, Altmann KH, McSheehy PMJ (2005) Patupilone (epothilone B, EPO906) and imatinib (STI571, Glivec) in combination display enhanced antitumour activity in vivo against experimental rat C6 glioma. Cancer Chemother Pharmacol 55:307–317
Obach RS (1999) Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metabo Dispos 27:1350–1359
Ogiu N, Nakamura Y, Ijiri I, Hiraiwa K, Ogiu T (1997) A statistical analysis of the internal organ weights of normal Japanese people. Health Phys 72:368–383
Parrott N, Paquereau N, Coassolo P, Lave T (2005) An evaluation of the utility of physiologically based models of pharmacokinetics in early drug discovery. J Pharma Sci 94:2327–2343
Potchoiba MJ, West M, Nocerini MR (1998) Quantitative comparison of autoradioluminographic and radiometric tissue distribution studies using carbon-14 labeled xenobiotics. Drug Metab Dispos 26:272–277
Poulin P, Jones RD, Jones HM, Gibson CR, Rowland M, Chien JY, Ring BJ, Adkison KK, Ku MS, He H, Vuppugalla R, Marathe P, Fischer V, Dutta S, Sinha VK, Bjornsson T, Lave T, Yates JW (2011) PHRMA CPCDC initiative on predictive models of human pharmacokinetics, part 5: Prediction of plasma concentration-time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J Pharma Sci 100:4147–4157
Poulin P, Theil FP (2002) Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharma Sci 91:129–156
Reese M, Sanchez-Pedregal VM, Kubicek K, Meiler J, Blommers MJJ, Griesinger C, Carlomagno T (2007) Structural basis of the activity of the microtubule-stabilizing agent epothilone A studied by NMR spectroscopy in solution. Angew Chem Int Ed Engl 46:1864–1868
Ring BJ, Chien JY, Adkison KK, Jones HM, Rowland M, Jones RD, Yates JW, Ku MS, Gibson CR, He H, Vuppugalla R, Marathe P, Fischer V, Dutta S, Sinha VK, Bjornsson T, Lave T, Poulin P (2011) PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 3: comparative assessment of prediction methods of human clearance. J Pharma Sci 94:1259–1276
Rodgers T, Leahy D, Rowland M (2005) Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharma Sci 94:1259–1276
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Rubin EH, Rothermel J, Tesfaye F, Chen T, Hubert M, Ho YY, Hsu CH, Oza AM (2005) Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J Clin Oncol 23:9120–9129
Tanabe KM, Millward M, Allen JD (2005) Interactions of patupilone (Epothilone B) with multidrug transporter proteins. Proc Amer Assoc Cancer Res 46 [Abstract] 3430 AACR meeting
Ten Bokkel Huinink WW, Sufliarsky J, Smit WM, Spanik S, Wagnerova M, Hirte HW, Kaye S, Johri AR, Oza AM (2009) Safety and efficacy of patupilone in patients with advanced ovarian, primary fallopian, or primary peritoneal cancer: a phase I, open-label, dose-escalation study. J Clin Oncol 27:3097–3103
Tsimberidou AM, Lewis N, Reid T, Burris H, Urban P, Tan EY, Anand S, Uehara C, Kurzrock R (2011) Pharmacokinetics and antitumor activity of patupilone combined with midazolam or omeprazole in patients with advanced cancer. Cancer Chemother Pharmacol 68:1507–1516
Zatloukal P, Mellemgaard A, Sanchez JM, Perry MC, Hamm JT, Van Meerbeeck J, Deleo JJ, Gao B, Johri AR, Felip E (2008) Activity of patupilone in advanced or metastatic non-small cell lung cancer (Nsclc): a phase II study. J Clin Oncol. 2007 ASCO annual meeting proceedings part I, vol 25, no 18S (June 20 Supplement), 18058 [abstract]
Acknowledgments
We thank Dr. Hilmar Schiller for conducting in vitro metabolism study and Dr. Swati Dumitras, Dr. Markus Zollinger, and Corinne Emotte for contributions in animal studies.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, B., Heimbach, T., Lin, Th. et al. Novel physiologically based pharmacokinetic modeling of patupilone for human pharmacokinetic predictions. Cancer Chemother Pharmacol 69, 1567–1582 (2012). https://doi.org/10.1007/s00280-012-1863-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1863-5