Abstract
Purpose
The pharmacokinetic profiles of bendamustine and active metabolites were defined in patients with rituximab-refractory, relapsed indolent B-cell non-Hodgkin’s lymphoma, and supported understanding of exposure–response relationships for efficacy and safety.
Methods
Bendamustine was administered as a 60-min 120 mg/m2 intravenous infusion on days 1 and 2 of six 21-day cycles. Pharmacokinetic models were developed, with covariate assessment. Correlations between bendamustine exposure and responder status or occurrence of neutropenia, thrombocytopenia, fatigue, nausea, and vomiting were examined.
Results
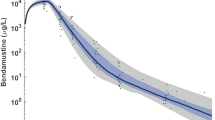
Following a single dose of bendamustine HCl, concentrations declined in a triphasic manner, with rapid distribution, intermediate, and slow terminal phases. The intermediate t 1/2 (40 min) was considered the pharmacologically relevant (beta elimination) t 1/2 since the initial phases accounted for 99% of the AUC. Age, sex, mild/moderate renal, or mild liver impairment did not alter pharmacokinetics. Metabolite concentrations were low relative to parent. No correlation was observed between exposure and safety or efficacy measures because of the limited range of exposures after 120 mg/m2 administration, except bendamustine C max was a significant (P value = 0.013) predictor of the probability of nausea in patients, most of whom were pretreated with antiemetics.
Conclusions
The BSA-based dosing regimen for bendamustine achieved the targeted exposure and was associated with a high incidence of therapeutic response. Given the short t 1/2 and low concentrations of bendamustine observed by 12 h after dosing, the single-dose profile for bendamustine described by these analyses is expected to be representative of the multiple-dose profile. The occurrence of nausea was significantly related to bendamustine exposure, with the probability of nausea increasing as bendamustine C max increases.






Similar content being viewed by others
References
Leoni LM, Bailey B, Reifert J et al (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other Alkylating agents. Clin Cancer Res 14:309–317
Strumberg D, Harstrick A, Doll K et al (1996) Bendamustine hydrochloride activity against doxorubicin-resistent human breast carcinoma cell lines. Anticancer Drugs 7:415–421
Friedberg JW, Cohen P, Chen L, Robinson KS, Forero-Torres A, La Casce AS et al (2008) Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 26:204–210
Bergmann MA (2006) Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CL Study Group. Haematologica 90:1357–1364
Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G et al (2006) Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone–a randomized phase III study of the East German study group of hematology and oncology (OSHO). J Cancer Res Clin Oncol 132:205–212
von Minckwitz G, Chernozemsky I, Sirakova L, Chilingirov P, Souchon R, Marschner N et al (2005) Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs 16:871–877
Schmittel A, Knodler M, Hortig P, Schulze K, Thiel E, Keilholz U (2007) Phase II trial of second-line bendamustine chemotherapy in relapsed small cell lung cancer patients. Lung Cancer 55:109–113
Teichert J, Baumann F, Chao Q, Franklin C, Bailey B, Hennig L et al (2007) Characterization of two phase I metabolites of bendamustine in human liver microsomes and in cancer patients treated with bendamustine hydrochloride. Cancer Chemother Pharmacol 59:759–770
Kahl BS, Bartlett NL, Leonard JP, Chen L, Ganjoo K, Williams ME, et al (2009) Bendamustine is effective therapy in patients with rituximab-refractory indolent B-cell non-Hodgkin’s lymphoma: results from a multicenter study. Cancer Nov 4 (Epub ahead of print)
World Health Organization (1998) Executive summary from the world symposium on primary pulmonary hypertension. World Health Organization, Evian, France
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
National Cancer Institute. Cancer Therapy Evaluation Program (2006) Common terminology criteria for adverse events v3.0 (CTCAE). http://www.ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 01 Aug 2007
Schwänen C, Hecker T, Hubinger G, Wolfle M, Rittgen W, Bergmann L et al (2002) In vitro evaluation of bendamustine induced apoptosis in B-chronic lymphocytic leukemia. Leukemia 16:2096–2105
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Beal SL, Sheiner LB, Boeckmann AJ (1989–2006) NONMEM users guides. Ellicott City, Maryland
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098
Peck CC, Conner DP, Murphy MG (1989) Bedside clinical pharmacokinetics: simple techniques for individualizing drug therapy. Vancouver, Washington
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Patel H, Egorin MJ, Remick SC, Mulkerin D, Takimoto CHM, Doroshow JH et al (2004) Comparison of Child-Pugh (CP) criteria and NCI organ dysfunction working group (NCI-ODWG) criteria for hepatic dysfunction (HD): Implications for chemotherapy dosing. J Clin Oncol 22(14S suppl):6051
Food and Drug Administration (1998) Guidance for industry pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Docket #97D-0214
Rasschaert M, Schrijvers D, Van den BJ, Dyck J, Bosmans J, Merkle K et al (2007) A phase I study of bendamustine hydrochloride administered day 1 + 2 every 3 weeks in patients with solid tumours. Br J Cancer 96:1692–1698
Rasschaert M, Schrijvers D, Dyck J, Bosmans J, Merkle K et al (2007) A phase I study of bendamustine hydrochloride administered once every 3 weeks in patients with solid tumors. Anticancer Drugs 18:587–595
Cephalon Inc (2009) Treanda (bendamustine) for injection. Cephalon Inc, Fraser PA (product information)
National Cancer Institute (2007) Fatigue (PDQ) health professional version. National Cancer Institute, Bethesda
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4:191–196
National Cancer Institute (2007) Nausea and vomiting (PDQ) health profession version. National Cancer Institute, Bethesda
Acknowledgments
Financial support for these analyses was provided by Cephalon, Incorporated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owen, J.S., Melhem, M., Passarell, J.A. et al. Bendamustine pharmacokinetic profile and exposure–response relationships in patients with indolent non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol 66, 1039–1049 (2010). https://doi.org/10.1007/s00280-010-1254-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1254-8