Abstract
Purpose
The clinical relevance of prolonged trastuzumab administration in combination therapy beyond progressive disease (PD) has been suggested. Here, we examined whether trastuzumab treatment is effective in combination after failing to show antitumor activity as monotherapy in HER2-positive human breast cancer xenograft models.
Methods
We established trastuzumab PD models with HER2-positive breast cancer xenograft models and compared the antitumor activity of trastuzumab in combination with a taxane versus monotherapy with a taxane in the models subsequent to tumor progression under trastuzumab monotherapy.
Results
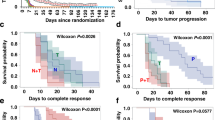
We established trastuzumab PD model using the HER2-positive human breast cancer line MDA-MB-361 and KPL-4 in in vivo. In these models, trastuzumab at the same dose as the initial treatment showed no significant antitumor activity at 3 weeks after start of treatment. Re-inoculated tumor tissues showing PD regained sensitivity to trastuzumab. In the trastuzumab PD models, the HER2 status of the tumor tissues did not decrease. Also, the pAKT level continued to decrease, as with the initial treatment, and IGF-1R was not found to be up-regulated. Instead, differences were observed in the gene-expression profiles of the tumor tissues showing PD. Trastuzumab in combination with G-CSF, which is expected to enhance antibody-dependent cellular cytotoxicity (ADCC), showed significant antitumor activity, even though the single agents alone showed no antitumor activity in the PD model. In the MDA-MB-361 trastuzumab PD model, the combination of trastuzumab with paclitaxel showed significantly more potent antitumor activity compared with paclitaxel or docetaxel monotherapy. In the KPL-4 trastuzumab PD model as well, trastuzumab showed significant antitumor activity in combination with taxanes or capecitabine after PD had developed in response to trastuzumab monotherapy.
Conclusion
We established in vivo trastuzumab PD models, in which trastuzumab monotherapy ceases to have antitumor activity during the treatment. The mechanisms of PD with trastuzumab are considered to involve both reversible changes in the gene expression profiles in tumor tissues and a decrease of ADCC activity in the host. Our present results demonstrated that trastuzumab showed antitumor activity in combination with taxanes or capecitabine even though it showed no antitumor activity as a monotherapy, suggesting a clinical relevance of treatment with trastuzumab as a combination therapy beyond PD.




Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, Mcguire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Austin CD, De Maziere AM, Pisacane PI et al (2004) Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell 15:5268–5282
Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL (2002) Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 62:4132–4141
Nagata Y, Lan KH, Zhou X et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6:117–127
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65:11118–11128
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6:443–446
Fujimoto-Ouchi K, Sekiguchi F, Tanaka Y (2002) Antitumor activity of combinations of anti-HER-2 antibody trastuzumab and oral fluoropyrimidines capecitabine/5′-dFUrd in human breast cancer models. Cancer Chemother Pharmacol 49:211–216
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Schaller G, Fuchs I, Gonsch T et al (2007) Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol 25:3246–3250
Cobleigh MA, Vogel CL, Tripathy D et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639–2648
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Kurebayashi J, Otsuki T, Tang CK et al (1999) Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer 79:707–717
Kunisue H, Kurebayashi J, Otsuki T et al (2000) Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer 82:46–51
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y (2007) Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol 59:795–805
Van Der Kolk LE, De Haas M, Grillo-Lopez AJ, JW Baars, Van Oers MH (2002) Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils. Leukemia 16:693–699
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J (1998) Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58:2825–2831
Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL (2007) Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13(16):4909–4919
Barok M, Isola J, Palyi-Krekk Z et al (2007) Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther 6:2065–2072
Kakinoki Y, Kubota H, Yamamoto Y (2004) CD64 surface expression on neutrophils and monocytes is significantly up-regulated after stimulation with granulocyte colony-stimulating factor during CHOP chemotherapy for patients with non-Hodgkin’s lymphoma. Int J Hematol 79:55–62
Bogdan C, Ding A (1992) Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J Leukoc Biol 52:119–121
Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H (1998) Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 4:1013–1019
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujimoto-Ouchi, K., Sekiguchi, F., Yamamoto, K. et al. Preclinical study of prolonged administration of trastuzumab as combination therapy after disease progression during trastuzumab monotherapy. Cancer Chemother Pharmacol 66, 269–276 (2010). https://doi.org/10.1007/s00280-009-1160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1160-0