Abstract
Purpose
Identification of a novel topoisomerase I inhibitor which shows superior efficacy and less individual variation than irinotecan hydrochloride (CPT-11).
Methods
A novel camptothecin analog that is effective against breast cancer resistance protein (BCRP)-positive cells was screened, and a water soluble prodrug was generated. Antitumor activity of the prodrug was examined in BCRP-positive and -negative xenografts both as a single agent and in combination with other anti-cancer drugs.
Results
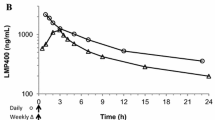
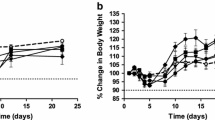
A novel camptothecin analog, CH0793076, was discovered. Because CH0793076 was found to be highly lipophilic, a water soluble prodrug (TP300) was generated. TP300 is stable in an acidic solution but is rapidly converted to CH0793076 under physiological pH conditions such as in sera. This efficient prodrug activation would minimize interpatient differences in pharmacokinetic and toxicity profiles. Unlike CPT-11, TP300 does not exhibit cholinergic interaction or cause acute diarrhea at effective doses. In mouse xenograft models, TP300 showed antitumor activity against both BCRP-positive and -negative xenografts, whereas CPT-11 was less active against BCRP-positive xenografts. In addition, the effective dose range (MTD/ED50) for TP300 was wider than for CPT-11 and TP300 showed additive or synergistic antitumor effects in combination with other anti-cancer drugs such as capecitabine, oxaliplatin, cisplatin, bevacizumab and cetuximab.
Conclusion
It is therefore expected that TP300 will provide an additional treatment option for patients who will undergo chemotherapy with camptothecins.









Similar content being viewed by others
References
de Jong FA, de Jonge MJA, Verweij J, Mathijssen RHJ (2006) Role of pharmacogenetics in irinotecan therapy. Cancer Lett 234:90–106
Saunders M, Iveson T (2006) Management of advanced colorectal cancer: state of the art. Br J Cancer 95:131–138
Fuchs C, Mitchell EP, Hoff PM (2006) Irinotecan in the treatment of colorectal cancer. Cancer Treat Rev 32:491–503
Markman M (2005) Topotecan as second-line therapy for ovarian cancer: dosage versus toxicity. Oncologist 10:695–697
Nicum SJ, O’Brien MER (2007) Topotecan for the treatment of small-cell lung cancer. Expert Rev Anticancer Ther 7:795–801
Mathijssen RHJ, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7:2182–2194
Gupta E, Mick R, Ramirez J, Wang X, Lestingi TM, Vokes EE, Ratain MJ (1997) Pharmacokinetic and pharmacodynamic evaluation of the topoisomerase inhibitor irinotecan in cancer patients. J Clin Oncol 15:1502–1510
de Jong FA, Mathijssen RHJ, Xie R, Verweij J, Sparreboom A (2004) Flat-fixed dosing of irinotecan: influence on pharmacokinetic and pharmacodynamic variability. Clin Cancer Res 10:4068–4071
Hecht JR (1998) Gastrointestinal toxicity or irinotecan. Oncology 12:72–78
Dodds HM, Rivory LP (1999) The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11). Mol Pharmacol 56:1346–1353
Ando Y, Saka H, Asai G, Sugiura S, Shimokata K, Kamataki T (1998) UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 9:845–847
Iyer L, Hall D, Das S, Mortell MA, Ramírez J, Kim S, Rienzo AD, Ratain MJ (1999) Phenotype–genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther 65:576–582
Ando Y, Ueoka H, Sugiyama T, Ichiki M, Shimokata K, Hasegawa Y (2002) Polymorphisms of UDP-glucuronosyltransferase and pharmacokinetics of irinotecan. Ther Drug Monit 24:111–116
Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2:43–47
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388
Marcuello E, Altés A, Menoyo A, del Rio E, Gómez-Pardo M, Baiget M (2004) UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer 91:678–682
Kehrer DFS, Sparreboom A, Verweij J, de Bruijn P, Nierop CA, van de Schraaf J, Ruijgrok EJ, de Jonge MJA (2001) Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res 7:1136–1141
Schellens JHM, Maliepaard M, Scheper RJ, Scheffer GL, Jonker JW, Smit JW, Beijnen JH, Schinkel AH (2000) Transport of topoisomerase I inhibitors by the breast cancer resistance protein. Potential clinical implications. Ann NY Acad Sci 922:188–194
Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, Shiozawa K, Kawabata S, Soda H, Ishikawa T, Tanabe S, Kohno S (2001) Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem Biophys Res Commun 288:827–832
Beretta GL, Perego P, Zunino F (2006) Mechanisms of cellular resistance to camptothecins. Curr Med Chem 13:3291–3305
Yang CH, Schneider E, Kuo ML, Volk EL, Rocchi E, Chen YC (2000) BCRP/MXR/ABCP expression in topotecan-resistant human breast carcinoma cells. Biochem Pharmacol 60:831–837
Özvegy C, Litman T, Szakács G, Nagy Z, Bates S, Váradi A, Sarkadi B (2001) Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun 285:111–117
Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y (2002) Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer 97:626–630
Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JHM, Scheper RJ, Germà-Lluch JR, Izquierdo MA (2002) Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 198:213–219
Ohwada J, Ozawa S, Kohchi M, Fukuda H, Murasaki C, Suda H, Murata T, Niizuma S, Tsukazaki M, Ori K, Yoshinari K, Itezono Y, Endo M, Ura M, Tanimura H, Miyazaki Y, Kawashima A, Nagao S, Namba E, Ogawa K, Kobayashi K, Okabe H, Umeda I, Shimma N (2009) Synthesis and biological activities of a pH-dependently activated water-soluble prodrug of a novel hexacyclic camptothecin analog. Bioorg Med Chem Lett 19:2772–2776
Teicher BA (2008) Next generation topoisomerase I inhibitors: rationale and biomarker strategies. Biochem Pharmacol 75:1262–1271
Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, Orsetti B, Scheffer GL, Ychou M, Khan QA, Pommier Y, Pau B, Martineau P, Del Rio M (2004) ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer 109:848–854
Bunting KD (2002) ABC Transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20:11–20
Acknowledgment
We thank F. Ford for proofreading the manuscript.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, M., Miwa, M., Ura, M. et al. A water soluble prodrug of a novel camptothecin analog is efficacious against breast cancer resistance protein-expressing tumor xenografts. Cancer Chemother Pharmacol 65, 363–371 (2010). https://doi.org/10.1007/s00280-009-1042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1042-5