Abstract
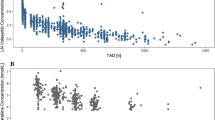
As pharmacokinetics represents a bridge between pharmacological concentrations and clinical regimens, the pharmacokinetic exploration of the therapeutic dose range is a major outcome. This study was aimed at assessing pharmacokinetic linearity of i.v. vinorelbine through an open design with intra-patient dose escalation (3 doses/group). Three groups of six patients received either 20–25–30 mg/m2; or 25–30–35 mg/m2; or 30–35–40 mg/m2. The inclusion criteria were: histologically confirmed tumour with at least one assessable target lesion, age 25–75 years, WHO PS ≤2, normal haematology and biochemistry, life expectancy ≥3 months. The pharmacokinetics was evaluated in both whole blood and plasma over 120 h. Twenty-six patients were recruited and 18 were evaluable for pharmacokinetics. The toxicity consisted in grade ≤3 leucopenia and neutropenia (<20% of courses) and two grade 4 constipation with rapid recovery (2/54 courses). Compared to blood, plasma was demonstrated to underestimate the pharmacokinetic parameters. In blood, the drug total clearance was about 0.6 l/h/kg, with minor contribution of renal clearance, steady state volume of distribution close to 13 l/h/kg, and elimination half-life at about 40 h. A pharmacokinetic linearity was demonstrated up to 40 mg/m2, and even up to 45 mg/m2 when pooling data from another study. A pharmacokinetic–pharmacodynamic relationship was evidenced on leucopenia and neutropenia when pooling the data from the two studies.








Similar content being viewed by others
References
Jacquillat CI, Khayat D, Weill UU, Band PR (1986) Les cancers—guide clinique, pronostique et thérapeutique. Edi Maloine
Chabner BA, Horwitz SB, Clendennin NJ, Purvis JP (1991) Vinca alkaloids. Cancer Chemother Biol Response Modif 12:67–73
Duflos A, Kruczynski A, Barret JM (2002) Novel aspects of natural and modified vinca alkaloids. Curr Med Chem Anti-Canc Agents 2(1):55–70
Corvalan JR, Smith W, Gore VA (1988) Tumour therapy with vinca alkaloids targeted by a hybrid–hybrid monoclonal antibody recognising both CEA and vinca alkaloids. Int J Cancer 2:22–25
Kruczynski A, Etievant C, Perrin D et al (2002) Characterization of cell death induced by vinflunine, the most recent vinca alkaloid in clinical development. Br J Cancer 86(1):143–150
Kruczynski A, Barret JM, Etievant C et al (1998) Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated vinca alkaloid. Biochem Pharmacol 55(5):635–648
Fedeli L, Colozza M, Boschetti E et al (1989) Pharmacokinetics of Vincristine in cancer patients treated with Nifedipine. Cancer 64(9):1805–1811
Focan C, Doalto L, Mazy V et al (1989) Vindesine en perfusion continue (suivie de Cisplatine) dans le cancer pulmonaire avancé Données chrono-pharmacocinétiques et efficacité clinique. Bull Cancer 76(8):909–912
Jackson DV, Sethi VS, Spurr CL et al (1981) Pharmacokinetics of Vincristine infusion. Cancer Treat Rep 65:1043–1046
Ohnuma T, Norter L, Andejazul A, Holland JF (1985) Pharmacokinetics of Vindesine given as an intravenous bolus and 24-hour infusion in humans. Cancer Res 45:464–469
Owellen RJ, Root MA, Hains FO (1977) Pharmacokinetics of Vindesine and Vincristine in Humans. Cancer Res 37:2603–2607
Pinkerton CR, Mcdermott B, Philip T et al (1988) Continuous Vincristine infusion as part of a high dose chemotherapy regimen drug kinetics and toxicity. Cancer Chemother Pharmacol 22:271–274
Rahmani R, Samak R, Bore P, Cano JP (1988) Pharmacocinétique clinique des vinca-alcaloides. Bull Cancer 75:195–200
Rahmani R, Gueritte F, Martin M et al (1986) Comparative pharmacokinetics of antitumor vinca alkaloïds: intravenous bolus injections of Navelbine and related alkaloïds to cancer patient and rats. Chemother Pharmacol 16:223–228
Bore P, Rahmani R, Van Confort J et al (1989) Pharmacokinetics of the new antitumor drug Navelbine in patients. Cancer Chemother Pharmacol 23:247–251
Rahmani R, Martin M, Barbet J, Cano JP (1984) Radioimmunoassay and preliminary pharmacokinetics studies in rats of 5′-Noranhydrovinblastine Navelbine. Cancer Res 44:5609–5613
Rahmani R, Bruno R, Iliadis A et al (1987) Clinical pharmacokinetics of the antitumor drug Navelbine (5′-Noranhydrovinblastine). Cancer Res 47:5796–5799
Krikorian A, Rahmani R, Bromet M et al (1989) Pharmacokinetics and metabolism of Navelbine. Semin Oncol 16(4):21–25
Solere P, Lucas C (1991) Limites analytiques en pharmacocinétique clinique: l’exemple des vinca-alcaloïdes. Bull Cancer 78:775–787
Mangeney P, Andriamialisoa RZ, Lallemand JY et al (1979) A new class of antitumor compounds: 5′-nor and 5′,6′-seco derivatives of vinblastine-type alkaloids. J Org Chem 44:3765–3768
Mangeney P, Andriamialisoa RZ, Lallemand JY et al (1979) 5′-noranhydrovinblastine prototype of a new class of vinblastine derivatives. Tetrahedron 35:2175–2179
Marty M, Extra JM, Espie M et al (1989) Advances in vinca-alkaloids: navelbine. Nouv Rev Fr Hematol 31:77–84
Hill BT (2001) Vinflunine, a second generation novel vinca alkaloid with a distinctive pharmacological profile, now in clinical development and prospects for future mitotic blockers. Curr Pharm Des 7(13):1199–1212
Fellous A, Ohayon R, Vacassin T et al (1989) Biochemical effects of navelbine on tubulin and associated proteins. Semin Oncol 16(4):9–14
Marquet P, Lachatre G, Debord J et al (1992) Pharmacokinetics of vinorelbine in man. Eur J Clin Pharmacol 42:545–547
Schilling T, Fiebig HH, Kerpel-Fronius S et al (1996) Clinical phase I and pharmacokinetic trial of vinorelbine administered as single intravenous bolus every 21 days in cancer patients. Invest New Drug 14:371–376
Wargin WA, Lucas VS (1994) The clinical pharmacokinetics of vinorelbine (Navelbine). Semin Oncol 21(5)Suppl 10:21–27
Leveque D, Jehl F, Quoix E, Monteil H (1993) Pharmacokinetic profile of vinorelbine, a new semi-synthetic vinca alkaloid, determined by high-performance liquid chromatography. Xenobiotica 23(11):1325–1333
Mathe G, Reizenstein P (1985) Phase I pharmacologic study of a new vinca alkaloid: navelbine. Cancer Lett 27:285–293
Krikorian A, Breillout F (1991) Vinorelbine (Navelbine), a new semisynthetic vinca alkaloid. Onkologie 14:7–12
Toso C, Lindley C (1995) Vinorelbine: a novel vinca alkaloid. Am J Health Syst Pharm 52:1287–1303
Crawford J, O’Rourke M, Schiller JH et al (1996) Randomized trial of vinorelbine compared with fluorouracil plus leucovorin in patients with stage IV non-small cell lung cancer. J Clin Oncol 14:2774–2784
Depierre A, Chastang C, Quoix E et al (1994) Vinorelbine versus vinorelbine plus cisplatin in advanced non-small cell lung cancer: a randomized trial. Ann Oncol 5:37–42
Furuse K, Kubota K, Kawahara M et al (1994) Japan vinorelbine lung cancer study group: a phase II study of vinorelbine, a new derivative of vinca alkaloid, for previously untreated advanced non-small cell lung cancer. Lung Cancer 11:385–391
Masotti A, Zannini G, Poggi R, Morandini GC (1994) Vinorelbine monochemotherapy in non-small-cell lung cancer: experience in patients with low performance status. Monaldi Arch Chest Dis 49:197–200
Depierre A, Lemarie E, Dabouis G et al (1991) A phase II study of navelbine (vinorelbine) in the treatment of non-small cell lung cancer. Am J Clin Oncol 14:115–119
Fossella FV, Devore R, Kerr RN et al (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens—the TAX 320 non-small cell lung cancer group. J Clin Oncol 18:2354–2362
Pronzato P, Landucci M, Vaira F et al (1994) Failure of vinorelbine to produce responses in pretreated non-small cell lung cancer patients. Anticancer Res 14:1413–1415
Rinaldi M, Della Giulia M, Venturo I et al (1994) Vinorelbine as single agent in the treatment of advanced non-small cell lung cancer. Proceedings of ASCO 1994; Abstract 1212, Dallas, USA
Santoro A, Maiorino L, Santoro M (1994) Second-line vinorelbine in the weekly monochemotherapy for the treatment of advanced non-small cell lung cancer. Lung Cancer 11(1):310
Vogel C, O’Rourke M, Winer E et al (1999) Vinorelbine as first-line chemotherapy for advanced breast cancer in women 60 years of age or older. Ann Oncol 10:397–402
Fumoleau P, Delgado FM, Delozier T et al (1993) Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol 11:1245–1252
Garcia-Conde J, Lluch A, Martin M et al (1994) Phase II trial of weekly iv vinorelbine in first-line advanced breast cancer chemotherapy. Ann Oncol 5:854–857
Bruno S, Puerto VL, Mickiewicz E et al (1995) Phase II trial of weekly iv vinorelbine as a single agent in first-line advanced breast cancer chemotherapy. The Latin-American experience. Am J Clin Oncol 18:392–396
Romero A, Rabinovich MG, Vallejo CT et al (1994) Vinorelbine as first-line chemotherapy for metastatic breast carcinoma. J Clin Oncol 12:336–341
Degardin M, Bonneterre J, Hecquet B et al (1994) Vinorelbine (navelbine) as a salvage treatment for advanced breast cancer. Ann Oncol 5:423–426
Gasparini G, Caffo O, Barni S et al (1994) Vinorelbine is an active antiproliferative agent in pretreated advanced breast cancer patients: a phase II study. J Clin Oncol 12:2094–2101
Fumoleau P, Delecroix V, Perrocheau G et al (1996) Final results of a phase I dose finding and pharmacokinetic (PK) study of docetaxel in combination with vinorelbine in metastatic breast cancer. Ann Oncol 7(5):126
Romero Acuna L, Langhi M, Perez J et al (1999) Vinorelbine and paclitaxel as first-line chemotherapy in metastatic breast cancer. J Clin Oncol 17(1):74–81
Goedhals L, Vorobiof DA, Bezwoda W et al (1999) Phase II trial of iv vinorelbine and cisplatin in inoperable locally advanced or disseminated non-small-cell lung cancer: the South African experience. Curr Med Res Opin 15(3):185–192
Isokangas OP, Knuuttila A, Halme M et al (1999) Phase II study of vinorelbine and gemcitabine for inoperable stage IIIB–IV non-small-cell lung cancer. Ann Oncol 10:1059–1063
Frasci G, Lorusso V, Panza N et al (2001) Gemcitabine plus vinorelbine yields better survival outcome than vinorelbine alone in elderly patients with advanced non-small cell lung cancer. A Southern Italy Cooperative Oncology Group (SICOG) phase III trial. Lung Cancer 34:S65–S69
Fujushita T, Loda M, Turner RE et al (2003) Sensitivity of non-small cell lung cancer cell lines established from patients treated with prolonged infusions of paclitaxel. Oncology 64(4):399–406
Rosen FR, Haraf DJ, Kies MS et al (2003) Multicenter randomised phase II study of paclitaxel (1-hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation without erythropoietin for advanced head and neck cancer. Clin Cancer Res 9(5):1689–1697
Henningsson A, Sparreboom A, Sandstrom M et al (2003) Population pharmacokinetic modelling of unbound and total plasma concentrations of paclitaxel in cancer patients. Eur J Cancer 39(8):1105–1114
Bissett D, Setanoians A, Cassidy J et al (1993) Phase I and pharmacokinetic study of taxotere (RP 56976) administered as a 24-hour infusion. Cancer Res 53:523–527
Extra JM, Rousseau F, Bruno R et al (1993) Phase I and pharmacokinetic study of taxotere (RP 56976; NSC 628503) administered as a short intravenous infusion. Cancer Res 53:1037–1042
Tomiak E, Piccart MJ, Kerger J et al (1994) Phase I study of docetaxel administered as a 1-hour intravenous infusion on a weekly basis. J Clin Oncol 12:1458–1467
Leveque D, Jehl F (1996) Clinical pharmacokinetics of vinorelbine. Clin Pharmacokinet 31(3):184–197
Leveque D, Quoix E, Dumont P et al (1993) Pulmonary distribution of vinorelbine in patients with non-small-cell lung cancer. Cancer Chemother Pharmacol 33:176–178
Zorza G, Guyomard C, Joly B et al (2000) In vitro metabolism of vinorelbine in rat, dog, and human. Proceedings of MDO 2000; Abstract 197, Stresa, Italy
Grudé P, Ratanasavanh D, Riché D et al (2000) Characterization of human cytochromes P450 isoenzymes involved in vinorelbine metabolism. Proceedings of MDO 2000; Abstract 179, Stresa, Italy
Focan C, Kreutz F, Leroy I et al (2001) Pharmacokinetics and mass-balance elimination of 3H-vinorelbine following IV and oral administration to patients. Proceedings of AACR 2001; Abstract 2064, New Orleans, USA
Soudon J, Zorza G, Van Heugen JC et al (2001) Search for vinorelbine metabolite activity: an in vitro cytotoxicity study using human ovary and lung cancer cell lines. Proceedings of AACR 2001; Abstract 2909, New Orleans, USA
Puozzo C, Zorza G, Guimbaud R et al (2000) Metabolism of vinorelbine in human: clinical application. Proceedings of AACR 2000; Abstract 1781, San Francisco, USA
Jehl F, Debs J (1990) Determination of navelbine and desacetyl-navelbine in biological fluids by high-performance liquid chromatography. J Chromatogr 525:225–233
Puozzo C, Gridelli C, Riggi M et al (2003) NSCLC in elderly patients: influence of age on NVB oral pharmacokinetics. Proceedings of AACR 2003; Abstract 920, Washington, USA
Puozzo C, Gridelli C, Jaworski M (2002) Pharmacokinetics of navelbine oral in elderly patients. Tumori 88(Suppl 1):75–76
Gridelli C, Guida C, Barletta E et al (2000) Thoracic radiotherapy and daily vinorelbine as radiosensitizer in locally advanced non-small cell lung cancer: a phase I study. Lung Cancer 29(2):131–137
Khayat D, Covelli A, Variol P et al (1995) Phase I and pharmacologic study of intravenous (Iv) vinorelbine in patients (PTS) with solid tumors. Proceedings of ASCO 1995; Abstract 1518, Los Angeles, USA
Urien S, Bree F, Breillout F et al (1993) Vinorelbine high affinity binding to human platelets and lymphocytes: distribution in human blood. Cancer Chemother Pharmacol 32:231–234
Marty M, Fumoleau P, Adenis A et al (2001) Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumours. Ann Oncol 12(11):1643–1649
Variol P, Bonneterre J, Marty M et al (2001) Pharmacokinetic/pharmacodynamic relationships of IV and oral Navelbine in phase I patients. Proceedings of ASCO 2001; Abstract 435, San Francisco, USA
Acknowledgements
The authors would also like to associate Drs Coiffier, Colombat, Gauthier and Rigal to this article for their contribution into patient enrolments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khayat, D., Rixe, O., Brunet, R. et al. Pharmacokinetic linearity of i.v. vinorelbine from an intra-patient dose escalation study design. Cancer Chemother Pharmacol 54, 193–205 (2004). https://doi.org/10.1007/s00280-004-0794-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0794-1