Abstract
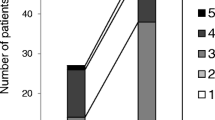
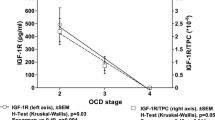
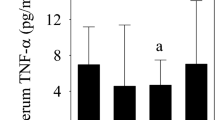
The possibility of controlling the harmful intra-articular influence of elevated interleukin (IL)-1β synovial fluid concentration after anterior cruciate ligament (ACL) surgery could be useful. We investigated the correlation between serum and synovial fluid IL-1β levels following ACL reconstruction. We measured IL-1β concentration periodically in three synovial fluid and four serum samples in each of 20 patients receiving either autologous conditioned serum (ACS) containing endogenous anti-inflammatory cytokines including IL-1Ra and several growth factors (group A) or placebo (group B). A decrease in IL-1β synovial fluid concentration appeared to be more pronounced in absolute terms in group A. In eight patients serum IL-1β was detected on the 6th postoperative day. In four of them whose synovial fluid levels were over 10 pg/ml on the 6th postoperative day, serum IL-1β was detected on the 10th postoperative day. The results were different in group B. Correlation between serum and synovial fluid IL-1β appearance persists in patients after ACL surgery and ACS application. This study is an example of ACS influence on the ACL healing process controlling the IL-1β levels on the basis of the serum IL-1β detection.
Résumé
Le contrôle de l’influence intra-articulaire néfaste de la concentration synoviale en l’IL-1β après chirurgie du croisé antérieur. Matériel et méthode: nous avons analysé les corrélations entre le sérum et le liquide synovial avec dosage des taux de l’IL-1β après la reconstruction du croisé antérieur. Nous avons mesuré la concentration de l’IL-1β de façon périodique avec trois prélèvements synoviaux et 4 dosages sériques chez 20 patients recevant un sérum ACS contenant des cytokines anti-inflammatoires incluant IL-1Ra et les différents facteurs de croissance (Groupe A) ou le Placébo (Groupe B). Résultats: la concentration de l’IL-1β dans le liquide synovial diminue de façon importante dans le groupe A. Chez 8 patients l’IL-1β sérique a été détectée au sixième jour post-opératoire. Pour chacun d’entre-eux le taux d’ IL-1β synovial était supérieur à 10 pg/ml, à la sixième heure post-opératoirel’IL-1β sérique a été détecté au 10ème jour post-opératoire. Les résultats sont très différents dans le groupe B. En conclusion, une corrélation persiste entre les taux sériques et les taux synoviaux de l’IL-1β chez les patients après chirurgie du croisé antérieur. Cette étude est un exemple de l’influence de l’ACS dans le processus de cicatrisation en contrôlant les taux de l’IL-1β et en analysant les taux sériques de celui-ci.


Similar content being viewed by others
References
Arend WP (1993) Interleukin-1 receptor antagonist. Adv Immunol 54:167–227
Berg EE, Pollard ME, Kang Q (2001) Interarticular bone tunnel healing. Arthroscopy 17:189–195
Borovecki F, Pecina-Slaus N, Vukicevic S (2007) Biological mechanisms of bone and cartilage remodelling—genomic perspective. Int Orthop 31:799–805
Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH (1997) The natural history of the anterior ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med 25:751–754
Cameron ML, Fu FH, Paessler HH, Schneider M, Evans CH (1994) Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc 2:38–44
Dinarello CA (1994) Blocking interleukin-1 receptors. Int J Clin Lab Res 24:61–79
Dinarello CA (1994) The interleukin-1 family: 10 years of discovery. FASEB J 8:1314–1325
Dinarello CA (2001) The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734
Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, Sheppard M, Krishnan BR, Pelletier JP (1999) In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol 154:1159–1169
Frank C, Amiel D, Woo SL, Akeson W (1985) Normal ligament properties and ligament healing. Clin Orthop Relat Res 196:15–25
Gonzalez C, Cava F, Ayllon A, Guevara P, Navajo JA, Gonzalez-Buitrago JM (2001) Biological variation of interleukin-1b, interleukin-8 and tumor necrosis factor-a in serum of healthy individuals. Clin Chem Lab Med 39:836–841
Granowitz EV, Clark BD, Mancilla J, Dinarello CA (1991) Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II intereleukin-1 receptor. J Biol Chem 266:14147–14150
Hoher J, Moller HD, Fu F (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc 6:231–240
Hundrić-Haspl Z, Pecina M, Haspl M, Tomicic M, Jukic I (2006) Plasma cytokines as markers of aseptic prosthesis loosening. Clin Orthop Relat Res 453:299–304
Jacobs JJ, Roebuck KA, Archibeck M et al (2001) Osteolysis: basic science. Clin Orthop 393:71–77
Irie K, Uchiyama E, Iwaso H (2003) Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10:93–96
Manologas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Marks P, Cameron M (2000) Inflammatory cytokine profiles correlate with the degree of chondrosis in the chronic anterior cruciate ligament deficient knee. ACL study meeting, Rhodes, Greece
Meijer H, Reinecke J, Becker C, Tholen G, Wehling P (2003) The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 52:404–407
Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S (2004) Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med 32:1619–1625
Poutsiaka DD, Clark BD, Vannier E, Dinarello CA (1991) Production of interleukin-1 receptor antagonist and interleukin-1 beta by peripheral blood mononuclear cells is differentially regulated. Blood 78:1275–1281
Volk HD, Keysser G, Burmester GR (1998) Cytokines and cytokine receptors. In: Thomas L (ed) Clinical laboratory diagnostics. Use and assessment of clinical laboratory results. TH-Books Verlagsgesellschaft, Frankfurt/Main, pp 764–773
Williams RJ 3rd, Hyman J, Petrigliano F, Rozental T, Wickiewicz TL (2004) Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. J Bone Joint Surg Am 86-A:225–232
Wilson TC, Kantaras A, Atay A, Johnson DL (2004) Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med 32:543–549
Zysk SP, Fraunberger P, Veihelmann A, Dorger M, Kalteis T, Maier M, Pellengahr C, Refior HJ (2004) Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc 12:98–103
Acknowledgement
The source of all funding for the study was obtained by the National Health Insurance.
We thank Vera Srsan Zivanovic (Department of General Surgery and Traumatology, General Hospital Varazdin), Damir Poljak (Department of Transfusion Medicine, General Hospital Varazdin) and Renata Jug (Institute of Immunology, Zagreb) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darabos, N., Hundric-Haspl, Z., Haspl, M. et al. Correlation between synovial fluid and serum IL-1β levels after ACL surgery–preliminary report. International Orthopaedics (SICOT) 33, 413–418 (2009). https://doi.org/10.1007/s00264-008-0649-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-008-0649-1