Abstract
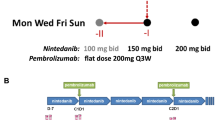
Modified FOLFOX6 is an established therapy for patients with metastatic colorectal cancer (mCRC). We conducted a single-arm phase Ib study to address the hypothesis that addition of pembrolizumab to this regimen could safely and effectively improve patient outcomes (NCT02375672). The relationship between immune biomarkers and clinical response were assessed in an exploratory manner. Patients with mCRC received concurrent pembrolizumab and modified FOLFOX6. The study included safety run-in for the first six patients. The primary objective was median progression-free survival (mPFS), with secondary objectives including median overall survival, safety, and exploratory assessment of immune changes. To assess immunological impact, peripheral blood was collected at baseline and during treatment. The levels of soluble factors were measured via bioplex, while a panel of checkpoint molecules and phenotypically defined cell populations were assessed with flow cytometry and correlated with RECIST and mPFS. Due to incidences of grade 3 and grade 4 neutropenia in the safety lead-in, the dose of mFOLFOX6 was reduced in the expansion cohort. Median PFS was 8.8 months and median OS was not reached at data cutoff. Best responses of stable disease, partial response, and complete response were observed in 43.3%, 50.0%, and 6.7% of patients, respectively. Several soluble and cellular immune biomarkers were associated with improved RECIST and mPFS. Immunosuppressive myeloid and T cell subsets that were analyzed were not associated with response. Primary endpoint was not superior to historic control. Biomarkers that were associated with improved response may be informative for future regimens combining chemotherapy with immune checkpoint inhibitors.





Similar content being viewed by others
Availability of data and material
All raw data and materials used to generate this manuscript and the included figures are available upon request from the corresponding author.
Code availability
Code utilized for the biostatistical analysis of this work is available from the corresponding author upon reasonable request.
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Van Cutsem E, Oliveira J, EGW Group (2009) Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(Suppl 4):61–63
SEER Program (National Cancer Institute (U.S.)) et al (1993) SEER cancer statistics review, in NIH publication., U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, Bethesda
Holch J, Stintzing S, Heinemann V (2016) Treatment of metastatic colorectal cancer: standard of care and future perspectives. Visc Med 32(3):178–183
Antoniotti C et al (2020) AtezoTRIBE: a randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer 20(1):683
Damato A et al (2020) Phase II study on first-line treatment of NIVolumab in combination with folfoxiri/bevacizumab in patients with Advanced COloRectal cancer RAS or BRAF mutated—NIVACOR trial (GOIRC-03-2018). BMC Cancer 20(1):822
de Gramont A et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Le DT et al (2020) Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 38(1):11–19
Le DT et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Weiss GJ et al (2017) A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer 117(1):33–40
Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Poulart V, Cullen D, Lei M, Kondo K, Li M, Ajani JA, Janjigian YY (2020) LBA6_PR-Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gasgroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of CheckMate649 study, in Ann Oncol
Galluzzi L et al (2017) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17(2):97–111
Panaretakis T et al (2009) Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28(5):578–590
Tesniere A et al (2010) Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29(4):482–491
Zaravinos A et al (2019) Cytolytic activity correlates with the mutational burden and deregulated expression of immune checkpoints in colorectal cancer. J Exp Clin Cancer Res 38(1):364
Zhou E et al (2015) Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol 8(7):8018–8027
Lines JL et al (2014) VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2(6):510–517
Kamphorst AO et al (2017) Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114(19):4993–4998
Meirow Y, Kanterman J, Baniyash M (2015) Paving the road to tumor development and spreading: myeloid-derived suppressor cells are ruling the fate. Front Immunol 6:523
Kanterman J et al (2014) Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res 74(21):6022–6035
Vincent J et al (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70(8):3052–3061
Wang Z, Till B, Gao Q (2017) Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 6(7):e1331807
Limagne E et al (2016) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res 76(18):5241–5252
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
National Cancer Institute (U.S.) (2009) Common terminology criteria for adverse events (CTCAE). Rev. ed. NIH publication, Bethesda, U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute
Toiyama Y et al (2012) Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int J Oncol 40(2):560–566
Chellappa S et al (2017) CD8+ T cells that coexpress RORgammat and T-bet are functionally impaired and expand in patients with distal bile duct cancer. J Immunol 198(4):1729–1739
Feun LG et al (2019) Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 125(20):3603–3614
Keegan A et al (2020) Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J Immunother Cancer 8(2):e000678
Mougiakakos D (2011) Regulatory T cells in colorectal cancer: from biology to prognostic relevance. Cancers (Basel) 3(2):1708–1731
Paz-Ares L et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Gandhi L et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Ozaslan E et al (2017) Efficacy and safety of cetuximab plus FOLFOX in second-line and third-line therapy in metastatic colorectal cancer. J BUON 22(4):863–868
Schultheis B et al (2013) Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Ann Oncol 24(6):1560–1567
Lee JJ, Sun W (2016) Options for second-line treatment in metastatic colorectal cancer. Clin Adv Hematol Oncol 14(1):46–54
Hao Q, Vadgama JV, Wang P (2020) CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal 18(1):82
Ramsey ML et al (2019) Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology 19(1):80–87
Kobelt D et al (2020) Pro-inflammatory TNF-alpha and IFN-gamma Promote Tumor Growth and Metastasis via Induction of MACC1. Front Immunol 11:980
Halama N et al (2016) Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29(4):587–601
Suenaga M et al (2019) Role of CCL5 and CCR5 gene polymorphisms in epidermal growth factor receptor signalling blockade in metastatic colorectal cancer: analysis of the FIRE-3 trial. Eur J Cancer 107:100–114
Llosa NJ et al (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5(1):43–51
Zhou G et al (2018) Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 7(7):e1448332
Ganesh K et al (2019) Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 16(6):361–375
Zeestraten EC et al (2013) The prognostic value of the apoptosis pathway in colorectal cancer: a review of the literature on biomarkers identified by immunohistochemistry. Biomark Cancer 5:13–29
Yu J et al (2021) Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 27(1):152–164
Acknowledgements
The authors are grateful for all the patients and families who participated in this clinical trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
Research funding for the clinical trial and correlative research studies was provided to investigators through a sponsored research agreement between Merck and Co., Inc and Emory University or The Ohio State University. Research reported in this publication was supported in part by the Cancer Tissue and Pathology shared resource and the Pediatrics/Winship Flow Cytometry Core of Winship Cancer Institute of Emory University and NIH/NCI under Award Number P30CA138292.
Author information
Authors and Affiliations
Contributions
GBL, BFE, and SS conceptualized this project and obtained funding for the execution. MRF and TAM generated the raw data for each figure. BO, TB, AN, CM, WS, CW, BFE, and SS conducted the clinical work associated with this trial. CJH, YT, and ZL analyzed the data. CJH wrote the original draft. All authors participated in revisions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MRF, CJH, YT, ZL, AN, CM, and TAM have no conflicts of interest to declare. BO has consulted for Genentech, Amgen, and Merck. The terms of these arrangements have been reviewed and approved by Indiana University in accordance with its conflict-of-interest policies. TBS has consulted for Ipsen, Array Biopharma, Pfizer, Seattle Genetics, Bayer, Genentech, Incyte, and Merck through his institution. TBS has also consulted for Boehringer Ingelheim, Janssen, Eisai, Daichii Sankyo, Natera, TreosBio, Celularity, Exact Science, and Sobi and has been compensated for these services. TBS has received research funding from Agios, Arys, Boston Biomedical, Bayer, Amgen, Merck, Celgene, Lilly, Ipsen, Clovis, Seattle Genetics, Array Biopharma, Genentech, Novartis, Mirati, Merus, Abgenomics, Incyte, Pfizer, and BMS. The terms of these arrangements have been reviewed and approved by Mayo Clinic in accordance with their conflict-of-interest policies. WS has received honoraria from Merck, BMS, Lexicon, Mylan, Ipsen, Blueprint Medicine, Signatera, and Exilexis. Dr. Shaib has also received research support from Lexicon, Eli Lilly, and GSK. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies. CW has consulted for Natera and Array Biopharma as well as received clinical trial funding from Vaccinex, Rapt Therapeutics, INBHRX, Boston Biomedical Inc., Seattle Genetics, Lycera, and Symphogen. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies. BFE has consulted for Ipsen, Merck and Co., Bayer, AstraZeneca, Bristol-Myers Squibb, Inc. and been a speaker for Lexicon, Inc. He serves as a consultant to Merck and Co., and receives compensation for these services. He has also received research funding through a sponsored research agreement between Emory University and Bristol-Myers Squibb, Boston Biomedical, Novartis, Merck and Co, Bayer, Exelixis, Pfizer, AstraZeneca/Medimmune, Incyte, and EUSA. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies. SS is currently employed by Eli Lilly. GBL has consulted for ProDa Biotech, LLC and received compensation. He has also received research funding through a sponsored research agreement between Emory University and Merck and Co., Bristol-Myers Squibb, Boerhinger-Ingelheim, and Vaccinex. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies.
Consent for publication
All authors and relevant funding sources have read and approved the final version of this manuscript and consent its publication.
Ethics approval and consent to participate
This study was conducted in accordance with the IRB procedures at Emory University (IRB00080442), Indiana University (1502701251), and The Ohio State University and was approved by their Ethics Boards. All patients were consented prior to participation in this trial (NCT02375672).This study was conducted in accordance with the IRB procedures at Emory University (IRB00080442), Indiana University (1502701251), and The Ohio State University and was approved by their Ethics Boards. All patients were consented prior to participation in this trial (NCT02375672).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Precis: We show that combining mFOLFOX and pembrolizumab is safe in patients with metastatic colorectal cancer. Correlative analyses identified immunotherapeutic targets, including LAG3, that warrant future investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herting, C.J., Farren, M.R., Tong, Y. et al. A multi-center, single-arm, phase Ib study of pembrolizumab (MK-3475) in combination with chemotherapy for patients with advanced colorectal cancer: HCRN GI14-186. Cancer Immunol Immunother 70, 3337–3348 (2021). https://doi.org/10.1007/s00262-021-02986-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02986-5