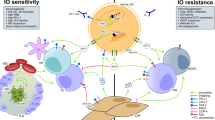
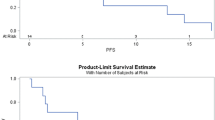
Abstract
Metformin has been widely used as the treatment of type II diabetes mellitus for its anti-hyperglycemic effect. In recent years, it has also been extensively studied for its anti-cancer effect as it diminishes immune exhaustion of CD8 + tumor-infiltrating lymphocytes (TILs). It decreases apoptosis of CD8 + TILs, thereby enhancing T cell-mediated immune response to tumor cells. Here, we present a unique case of a patient with small cell lung cancer (SCLC) who exhibited an overall partial response as per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) since starting metformin in combination with nivolumab therapy. Our patient had been treated with nivolumab monotherapy for 2 years until she had progression of disease. After she was started on metformin along with nivolumab therapy, she has shown a significant durable response for over 6 months. Many patients develop resistance to immunotherapy such as antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). Tumor hypoxia is one of the resistance factors. Signals activated by hypoxic environments in tumors are associated with decreased sensitivity to the PD-1 blockade. Metformin inhibits oxygen consumption in tumor cells in vitro and in vivo, reducing intratumoral hypoxia. These data suggest that metformin can improve susceptibility to anti-PD-1 treatment. To the best of our knowledge, our case is the first reported example demonstrating a proof-of-concept that metformin can contribute to overcoming acquired resistance to PD-1 inhibitors.


Similar content being viewed by others
Code availability
Not applicable.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- CT:
-
Computed tomography
- ED:
-
Extensive-disease
- ICIs:
-
Immune checkpoint inhibitors
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death ligand 1
- RECIST 1.1:
-
Response Evaluation Criteria in Solid Tumors, version 1.1
- SCLC:
-
Small cell lung cancer
- TILs:
-
Tumor-infiltrating lymphocytes
References
Chae YK et al (2016) Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 7(26):40767–40780
Scharping NE et al (2017) Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res 5(1):9–16
Viveiros P et al (2019) EP1.04-12 response to combination of metformin and nivolumab in a NSCLC patient whose disease previously progressed on nivolumab. J Thor Oncol 14(10):S976
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Wang S et al (2017) Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 7(1):1339
Paz-Ares L et al (2019) Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212):1929–1939
Horn L et al (2018) First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New Engl J Med 379(23):2220–2229
Antonia SJ et al (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17(7):883–895
Horn L et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229
Reck M et al (2018) Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol 1:29
Owonikoko TK et al (2019) Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study. Ann Oncol 30:77
Tucker N (2020) Pembrolizumab plus chemotherapy phase III study shows mixed results in sclc, in target oncology. Oncology 3:6
Pavan A et al (2019) Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J ImmunoThera Cancer 7(1):205
Chae YK, Oh MS, Giles FJ (2018) Molecular biomarkers of primary and acquired resistance to T-cell-mediated immunotherapy in cancer: landscape, clinical implications, and future directions. Oncol 23(4):410–421
Zhou X et al (2016) Metformin suppresses hypoxia-induced stabilization of HIF-1 alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget 7(1):873–884
Joshua AM et al (2014) A pilot ‘window of opportunity’ neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dis 17(3):252–258
Camacho L, Dasgupta A, Jiralerspong S (2015) Metformin in breast cancer-an evolving mystery. Breast Cancer Res 17(1):88. https://doi.org/10.1186/s13058-015-0598-8
Acknowledgements
Andrew Alexander is acknowledged for providing pictures of Fig. 1 and writing captions.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YK: writing and revising the manuscript content. YKC: supervision and validation. Others: review and editing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
Young Kwang Chae—Research Grant: Abbvie, BMS, Biodesix, Lexent Bio, and Freenome; Honoraria/Advisory Boards: Roche/Genentech, AstraZeneca, Foundation Medicine, Counsyl, Neogenomics, Guardant Health, Boehringher Ingelheim, Biodesix, Immuneoncia, Lilly Oncology, Merck, Takeda, Pfizer, and Tempus.
Ethical approval
We have obtained written informed consent from the patient for the publication of the case report.
Availability of data and material
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Consent for publication
We have obtained written informed consent from the patient for the publication of the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y., Vagia, E., Viveiros, P. et al. Overcoming acquired resistance to PD-1 inhibitor with the addition of metformin in small cell lung cancer (SCLC). Cancer Immunol Immunother 70, 961–965 (2021). https://doi.org/10.1007/s00262-020-02703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02703-8