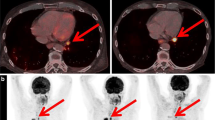
Abstract
Purpose
This prospective study aimed (1) to assess the non-small cell lung cancer (NSCLC) evolutive patterns to immunotherapy using FDG-PET and (2) to describe their association with clinical outcome.
Design
Fifty patients with metastatic NSCLC were included before pembrolizumab or nivolumab initiation. FDG-PET scan was performed at baseline and after 7 weeks of treatment (PETinterim1) and different criteria/parameters of tumor response were assessed, including PET response criteria in solid tumors (PERCIST). If a first PERCIST progressive disease (PD) without clinical worsening was observed, treatment was continued and a subsequent FDG-PET (PETinterim2) was performed at 3 months of treatment. Pseudo-progression (PsPD) was defined as a PERCIST response/stability on PETinterim2 after an initial PD. If a second PERCIST PD was assessed on PETinterim2, a homogeneous progression of lesions (termed immune homogeneous progressive-disease: iPDhomogeneous) was distinguished from a heterogeneous evolution (termed immune dissociated-response: iDR). A durable clinical benefit (DCB) of immunotherapy was defined as treatment continuation over a 6-month period. The association between PET evolutive profiles and DCB was assessed.
Results
Using PERCIST on PETinterim1, 42% (21/50) of patients showed a response or stable disease, most of them (18/21) reached a DCB. In contrast, 58% (29/50) showed a PD, but more than one-third (11/29) were misclassified as they finally reached a DCB. No standard PETinterim1 criteria could accurately distinguished responding from non-responding patients. Treatment was continued in 19/29 of patients with a first PERCIST PD; the subsequent PETinterim2 demonstrated iPDhomogeneous, iDR and PsPD in 42% (8/19), 26% (5/19), and 32% (6/19), respectively. Whereas no patients with iPDhomogeneous experienced a DCB, all patients with iDR and PsPD reached a clinical benefit to immunotherapy.
Conclusion
In patients with a first PD on PERCIST and treatment continuation, a subsequent PET identifies more than half of them with iDR and PsPD, both patterns being strongly associated with a clinical benefit of immunotherapy.




Similar content being viewed by others
References
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer [Internet]. https://doi.org/10.1056/NEJMoa1504627. 2015 [cited 2019 Mar 6]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1504627.
Catacchio I, Scattone A, Silvestris N, Mangia A. Immune prophets of lung cancer: the prognostic and predictive landscape of cellular and molecular immune markers. Transl Oncol. 2018;11:825–35.
Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non–small cell lung cancer. Lung Cancer Amst Neth. 2016;99:79–87.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med Off Publ Soc Nucl Med. 2009;50(Suppl 1):122S–50S.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy : report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46:238–50.
Shields AF, Jacobs PM, Sznol M, Graham MM, Germain RN, Lum LG, et al. Immune modulation therapy and imaging: workshop report. J Nucl Med. 2018;59:410–7.
Carter BW, Bhosale PR, Yang WT. Immunotherapy and the role of imaging. Cancer. 2018;124:2906–22.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Leiserson MDM, Syrgkanis V, Gilson A, Dudik M, Gillett S, Chayes J, et al. A multifactorial model of T cell expansion and durable clinical benefit in response to a PD-L1 inhibitor. PLoS ONE [Internet]. 2018 [cited 2019 Mar 8];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6312275/.
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47.
Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U, et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45:1289–96.
Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9:8.
Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med. 2007;48:36S–44S.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Higuchi M, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, et al. FDG-PET in the evaluation of response to nivolumab in recurrent non-small-cell lung cancer. World J Surg Oncol [Internet]. 2016 [cited 2019 Mar 8];14. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5011843/.
Cho SY, Lipson EJ, Im H-J, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96.
Wang GX, Kurra V, Gainor JF, Sullivan RJ, Flaherty KT, Lee SI, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiogr Rev Publ Radiol Soc N Am Inc. 2017;37:2132–44.
Hodi FS, Hwu W-J, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7.
Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer [Internet]. 2016 [cited 2019 Mar 8];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5168591/.
Hammer M, Bagley S, Aggarwal C, Bauml J, Nachiappan AC, Simone CB, et al. Thoracic imaging of non-small cell lung cancer treated with anti-programmed death receptor-1 therapy. Curr Probl Diagn Radiol. 2019;48:142–7.
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52.
Vrankar M, Unk M. Immune RECIST criteria and symptomatic pseudoprogression in non-small cell lung cancer patients treated with immunotherapy. Radiol Oncol. 2018;52:365–9.
Deuschl C, Nensa F, Grueneisen J, Poeppel TD, Sawicki LM, Heusch P, et al. Diagnostic impact of integrated 18F-FDG PET/MRI in cerebral staging of patients with non-small cell lung cancer. Acta Radiol Stockh Swed 1987. 2017;58:991–6.
Nia ES, Garland LL, Eshghi N, Nia BB, Avery RJ, Kuo PH. Incidence of brain metastases on follow-up 18F-FDG PET/CT scans of non-small cell lung cancer patients: should we include the brain? J Nucl Med Technol. 2017;45:193–7.
Acknowledgements
The authors would like to thank Colin Debaigt for protocol submission to regulatory agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O. Humbert, N. Cadour, M. Paquet, R. Schiappa, M. Poudenx, D. Chardin, D. Borchiellini, D. Benisvy, M.J. Ouvrier, C. Zwarthoed, A. Schiazza, H. Ghalloussi, P.M. Koulibaly, J. Darcourt, and J. Otto declare that they have no conflict of interest. M. Ilie reports personal fees from AstraZeneca, Bristol-Myers Squibb, Roche, Boehringer-Ingelheim, and Merck & Co outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Chest
Rights and permissions
About this article
Cite this article
Humbert, O., Cadour, N., Paquet, M. et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging 47, 1158–1167 (2020). https://doi.org/10.1007/s00259-019-04573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04573-4