Abstract
Purpose
Cetuximab, a chimeric monoclonal antibody targeting epidermal growth factor receptor (EGFR) on the surface of cancer cells, was approved by the FDA to treat patients with metastatic colorectal cancer. It is currently also in advanced-stage development for the treatment of several other solid tumors. Here we report for the first time the quantitative positron emission tomography (PET) imaging of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab.
Methods
We conjugated cetuximab with macrocyclic chelating agent 1,4,7,10-tetraazadodecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA), labeled with 64Cu, and tested the resulting 64Cu-DOTA-cetuximab in seven xenograft tumor models. The tracer uptake measured by PET was correlated with the EGFR expression quantified by western blotting. The estimated human dosimetry based on the PET data in Sprague-Dawley rats was also calculated.
Results
MicroPET imaging showed that 64Cu-DOTA-cetuximab had increasing tumor activity accumulation over time in EGFR-positive tumors but relatively low uptake in EGFR-negative tumors at all times examined (<5%ID/g). There was a good correlation (R 2 = 0.80) between the tracer uptake (measured by PET) and the EGFR expression level (measured by western blotting). Human dosimetry estimation indicated that the tracer may be safely administered to human patients for tumor diagnosis, with the dose-limiting organ being the liver.
Conclusion
The success of EGFR-positive tumor imaging using 64Cu-DOTA-cetuximab can be translated into the clinic to characterize the pharmacokinetics, to select the right population of patients for EGFR-targeted therapy, to monitor the therapeutic efficacy of anti-EGFR treatment, and to optimize the dosage of either cetuximab alone or cetuximab in combination with other therapeutic agents.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) is a member of the erbB family of tyrosine kinase receptors [1]. It is a 170-kDa cell surface protein composed of three regions: an intracellular domain with adenosine triphosphatase (ATP)-dependent tyrosine kinase activity, a hydrophobic transmembrane domain, and an extracellular ligand binding domain [2]. Many epithelial cancers over-express EGFR, and dysregulation of EGFR is associated with several key features of cancer such as autonomous cell growth, inhibition of apoptosis, angiogenic potential, invasion, and metastases [3, 4].
Many monoclonal antibody (mAb) products have been approved for clinical use in the United States, and dozens more are currently in clinical evaluation [5, 6]. Cetuximab (Erbitux; C255; ImClone, Somerville, NJ and Bristol-Myers Squibb, New York, NY), an immunoglobulin G1 mouse–human chimeric monoclonal antibody that binds with high affinity to EGFR, was the first anti-EGFR mAb to be evaluated clinically [7]. By blocking the EGFR signal transduction pathway, cetuximab can cause G1 phase cell cycle arrest and disruption of cell cycle progression which occurs through inhibition of DNA repair mechanisms and survival pathways, decrease in angiogenesis and cellular adhesion, and inhibition of matrix metalloproteinases required for metastatic invasion [8]. It was recently approved by FDA for the treatment of patients with EGFR-expressing metastatic colorectal cancer. Cetuximab (either alone or in combination with other treatment modalities) is also currently in advanced clinical trials in several other types of solid tumor, such as head and neck cancer [9], non-small cell lung cancer (NSCLC) [10], pancreatic cancer [11], cervical cancer, recurrent epithelial ovarian cancer, primary peritoneal carcinoma, and rectal cancer [12].
99mTc-labeled cetuximab has been reported for single-photon emission computed tomography (SPECT) imaging of EGFR expression [13]. This tracer has reasonable dosimetric properties for a diagnostic nuclear medicine agent. The potential of the long-lived positron emitter 89Zr in predicting the biodistribution of therapeutic radiometals (such as 88Y, 90Y, and 177Lu) when labeled to cetuximab via different types of chelator has been evaluated [14]. 68Ga-DOTA-hEGF has also been reported for EGFR imaging [15].
Since many cancer types over-express EGF and EGFR, detection of EGFR over-expression can provide important diagnostic information, which can influence patient management. For positron emission tomography (PET) imaging of EGFR expression, 64Cu-labeled mAb is of particular interest since the half-life of 64Cu (12.7 h) is suitable for the targeting and clearance kinetics of the mAb [16]. Although cetuximab is currently in the clinic, no quantitative PET imaging using 64Cu-labeled cetuximab has been reported. Quantitative non-invasive measurement of EGFR expression level in vivo is critical in the patient selection for cetuximab treatment or clinical trials and monitoring of treatment response. Herein we describe the use of 64Cu-labeled cetuximab for PET imaging of EGFR expression in vivo, and the tracer uptake measured by PET scans was correlated with the EGFR expression measured by western blotting.
Materials and methods
All commercially available chemical reagents were used without further purification, 1,4,7,10-Tetraazadodecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA) was purchased from Macrocyclics, Inc. (Dallas, TX) and Chelex 100 resin (50–100 mesh) was purchased from Aldrich (St. Louis, MO). Water and all buffers were passed through Chelex 100 column (1 × 15 cm) before use in radiolabeling procedures to ensure that the aqueous buffer is heavy metal free. PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NJ). Athymic nude mice were obtained from Harlan (Indianapolis, IN) at 4–5 weeks of age. 64Cu was provided by Washington University in St. Louis and University of Wisconsin, Madison.
Cell lines and xenografts
The U87MG human glioblastoma, PC-3 human prostate carcinoma, CT-26 murine colorectal carcinoma, HCT-8, HCT-116, and SW620 human colorectal carcinoma, and MDA-MB-435 human breast cancer cell lines were obtained from the American Type Culture Collection (ATCC) and maintained under standard conditions [17–19]. All animal experiments were performed under a protocol approved by the Stanford University Administrative Panel on Laboratory Animal Care (A-PLAC). All subcutaneous tumor models were established in 4- to 6-week-old female athymic nude mice except for the PC-3 tumor, where male mice were used. Typically 5 × 106 cells suspended in 100 μl serum-free corresponding culture medium (Invitrogen Inc., Carlsbad, CA) were injected into the front flank of the mice. MDA-MB-435 breast cancer model was established by orthotopic injection of 5 × 106 cells into the mammary fat pad of female athymic nude mice [20]. The mice were used for microPET imaging studies when the tumor volume reached 200–400 mm3 (1–4 weeks after inoculation of the cancer cells).
DOTA conjugation and radiolabeling
The detailed procedure for DOTA conjugation was reported earlier [21, 22]. The synthetic scheme of DOTA-cetuximab is shown in Fig. 1. 64CuCl2 (74 MBq) was diluted in 300 μl of 0.1 M sodium acetate buffer (pH 6.5) and added to 50 μg of DOTA-cetuximab. The reaction mixture was incubated for 1 h at 40°C with constant shaking. 64Cu-DOTA-cetuximab was then purified by PD-10 column using PBS as the mobile phase.
Number of DOTA per cetuximab and immunoreactive fraction
The average number of DOTA chelators per cetuximab was determined using a previously reported procedure with slight modification [23, 24]. Briefly, a defined amount of non-radioactive CuCl2 (80-fold excess of DOTA-cetuximab) in 40 μl 0.1 N NaOAc buffer was added to 37 MBq of 64CuCl2 in 20 μl 0.1 N NaOAc buffer, and 20 μg of DOTA-cetuximab in 100 μl 0.1 N NaOAc buffer was added to the above carrier-added 64CuCl2 solution. After purification using PD-10 column, the number of DOTA per cetuximab was calculated using the following equation:
The result was expressed as mean±SD (n = 3). The immunoreactive fraction of 64Cu-DOTA-cetuximab was determined by incubation with U87MG cells (EGFR positive and exhibited the higher tracer uptake in the seven tumor models) in suspension culture under conditions of antigen excess (n = 3) [25]. The immunoreactive fraction was calculated as:
MicroPET and image analysis
Details of the PET procedure have been reported earlier [26, 27]. Briefly, PET scans were performed using a microPET R4 rodent model scanner (Concorde Microsystems Inc., Knoxville, TN). Scanner calibration was performed to map between microPET image units and units of radioactivity concentration. Mice were injected with 5–10 MBq of 64Cu-DOTA-cetuximab via the tail vein under isoflurane anesthesia, and 2–20 min static PET scans were performed at various time points post injection (p.i.). The images were reconstructed by a two-dimensional ordered subsets expectation maximum algorithm and no correction was applied for attenuation or scatter [28]. For each microPET scan, three-dimensional regions of interests (ROIs) were drawn over the tumor and major organs by using vendor software ASI Pro 5.2.4.0 on decay-corrected whole-body coronal images. Values in three to ten adjacent slices (depending on the size of the tumor or organ) were averaged to obtain a reproducible value of activity concentration in the ROI drawn. Assuming a tissue density of 1 g/ml, the ROIs were converted to MBq/g per minute using a conversion factor, and then divided by the administered activity to obtain an imaging ROI-derived percent injected dose per gram of tissue (%ID/g).
Western blot
After the microPET studies and after the radioactivity had mostly decayed, the mice were sacrificed and the tumor tissues were harvested. Tumor tissue protein was extracted using T-PER tissue protein extraction buffer and the concentration was determined using microBCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL). After SDS-PAGE separation of 40 μg of total protein, it was transferred to a polyvinylidene fluoride membrane (Invitrogen Corp., Carlsbad, CA) and incubated at room temperature with 5% non-fat milk blocking buffer. The blots were then incubated overnight at 4°C with cetuximab, followed by incubation at room temperature for 1 h with HRP-conjugated anti-human antibody (GE Healthcare, Piscataway, NJ). The bands were detected using the ECL western blotting detection system (GE Healthcare). Tubulin was used as loading control. After development, films were scanned with a scanner using grayscale mode. The images were opened in ImageJ, a Java-based image processing software. An ROI was drawn over the first lane, and was applied for other lanes and the background to obtain densitometric signal from equal area. Background signal was subtracted before normalization. Three samples of each tumor model were prepared for western blot to obtain semiquantitative data for statistical analysis.
Immunofluorescence staining
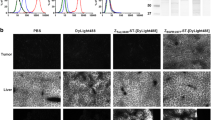
Two representative tumors (PC-3 and MDA-MB-435, with high and low tracer uptake, respectively) were chosen for qualitative evaluation of the tumor EGFR expression. Frozen tumor sections (5 μm thick) were warmed to room temperature, fixed with ice-cold acetone for 10 min, and then dried in the air for 30 min. The sections were rinsed in PBS for 2 min and blocked in 10% donkey serum for 1 h at room temperature. The sections were incubated with cetuximab (100 μg/ml) for 1 h at room temperature and visualized with FITC-conjugated donkey anti-human secondary antibody (1:200, Jackson ImmunoResearch Laboratories, Inc.) under a microscope (Carl Zeiss Axiovert 200M, Carl Zeiss USA, Thornwood, NY). Images were taken under the same condition and displayed at the same scale.
Radiation dosimetry extrapolation to human
Estimated human dosimetry was calculated from microPET imaging results on Sprague-Dawley female rats (Harlan) injected with about 37 MBq of 64Cu-DOTA-cetuximab. The rats were scanned at two bed positions in order to cover the whole body, and ROI analysis was carried out on major organs [22, 24, 29]. Time-activity curves were generated from the mean values obtained in rats for each organ of interest. We then calculated source organ residence times for the human model by integrating a monoexponential fit to the experimental biodistribution data for major organs and the whole body. The source organ residence times obtained forthwith were used with a standard quantitation platform Organ Level Internal Dose Assessment (OLINDA; Vanderbilt University) [30]. Projected radiation absorbed doses for humans were made by assuming that, for the purpose of this exercise, the metabolism rates and pharmacokinetics of 64Cu-DOTA-cetuximab in man and mouse are equivalent. Therefore, the biodistribution data were assumed to apply to a reference adult human subject. The whole-body time-activity curve was integrated by fitting the whole-body retention data to a biphasic exponential decay curve, which represented the retention data more closely than a single exponential equation.
Statistical analysis
Quantitative data were expressed as mean±SD. Means were compared using one-way ANOVA and Student’s t test. P values <0.05 were considered statistically significant.
Results
Radiolabeling yield, specific activity, number of DOTA per cetuximab, and immunoreactive fraction
The radiolabeling procedure took about 85 ± 10 min (n = 5) and the radiochemical yield was 84.0 ± 8.7% (based on 74 MBq of 64Cu per 50 μg of DOTA-cetuximab). The specific activity of 64Cu-DOTA-cetuximab was 1.24 ± 0.13 GBq/mg cetuximab. 64Cu-DOTA-cetuximab was determined to have 21.5 ± 2.2 DOTA residues per cetuximab (n = 4). Using U87MG cells, the immunoreactive fraction was measured to be 62.6 ± 13.7%, indicating that the accessible lysine residues were largely located away from the complementarity-determining region.
MicroPET of tumor models with different EGFR expression
As can be seen in Fig. 2, the tumor uptake of 64Cu-DOTA-cetuximab was significantly higher in U87MG and PC-3 tumors (high EGFR expression) than in the other tumors except at 1 h p.i., when the injected tracer was still mainly in the blood pool. The uptake of 64Cu-DOTA-cetuximab in the major organs and tissues was similar in all seven tumor models tested (Fig. 3a). The blood and liver activity was high at early time points owing to non-specific uptake of the tracer by the reticuloendothelial system but dropped steadily over time, while the tracer uptake in all other organs was at a very low level (less than 3.5%ID/g). For the blood, the activity concentration was 20.9 ± 2.5, 9.4 ± 1.2, and 5.7 ± 1.9%ID/g at 1, 16, and 48 h p.i., respectively (n = 6), indicating that the serum half-life of the tracer was less than 18 h in the mouse. For the liver, the uptake was 19.7 ± 2.7, 14.0 ± 1.4, and 12.5 ± 2.5%ID/g at 1, 16, and 48 h p.i., respectively (n = 6). 64Cu-DOTA-cetuximab was mainly cleared through the hepatic pathway and virtually no renal uptake or renal clearance was observed. The uptake of 64Cu-DOTA-cetuximab in the EGFR-positive tumors increased with time and reached a plateau at around 24 h p.i. (Fig. 3b). For the U87MG tumor, the uptake was 3.6 ± 0.3, 10.3 ± 1.1, and 13.2 ± 1.1%ID/g at 1, 16, and 48 h p.i., respectively. For the PC-3 tumor, the uptake was 3.7 ± 0.6, 9.2 ± 3.1, and 12.8 ± 1.6%ID/g at 1, 16, and 48 h p.i., respectively. The uptake of 64Cu-DOTA-cetuximab in the low EGFR-expressing tumors was low at all time points examined (Fig. 3b). For the SW620 tumor, the uptake was 1.8 ± 0.1, 4.4 ± 0.6, and 3.9 ± 0.6%ID/g at 1, 16, and 48 h p.i., respectively. For the MDA-MB-435 tumor, the uptake was 2.5 ± 0.5, 3.0 ± 0.3, and 2.7 ± 0.5%ID/g at 1, 16, and 48 h p.i., respectively. The 64Cu-DOTA-cetuximab uptake in the CT-26 tumor decreased after 18 h p.i., likely owing to the very fast growing nature of the tumor (the tumor volume almost doubled over the 48-h time span of the study). An ROI was drawn on the hind limb of the tumor-bearing mice (muscle) to measure the muscle uptake of the tracer. Since the activity accumulation in the muscle is very low, the %ID/g values of the muscle were calculated to be between 0.5 and 3.0%ID/g (Fig. 3a).
a Time-activity curves for the blood, liver, muscle, and kidney uptake of 64Cu-DOTA-cetuximab (n = 6). b Time-activity curves of 64Cu-DOTA-cetuximab uptake in the seven tumor models (n = 3 per model). c Time-activity curves of major organ uptake of 64Cu-DOTA-cetuximab in Sprague-Dawley female rats (n = 3)
Correlation of 64Cu-DOTA-cetuximab uptake with EGFR expression level
From the PET scans, it was found that U87MG and PC-3 tumors have high EGFR expression, CT-26 and HCT-8 tumors have medium EGFR expression, while HCT-116, SW620, and MDA-MB-435 tumors have low EGFR expression. In order to quantify the EGFR expression level in vivo, western blot of each tumor tissue protein extract (n = 3 per tumor model, except for MDA-MB-435, where n = 1) were carried out and the EGFR band intensity was quantified (Fig. 4a). Since the PET scans revealed that the tumor uptake reached a plateau after 24 h, the %ID/g values at 48 h p.i. were used to correlate with the EGFR expression level measured by western blotting (Fig. 5a). A linear relationship was found between the %ID/g values and the EGFR band intensity (R 2 = 0.80). The tumor-to-muscle ratio at 48 h p.i. also showed a linear correlation with the EGFR band intensity (R 2 = 0.64) (Fig. 5b). The EGFR specificity of 64Cu-DOTA-cetuximab uptake in vivo was further confirmed by immunofluorescence staining. Using cetuximab as the primary antibody, PC-3 tumor (high 64Cu-DOTA-cetuximab uptake) showed strong EGFR staining and MDA-MB-435 tumor (low 64Cu-DOTA-cetuximab uptake) had only weak and patchy staining (Fig. 4b).
a Western blot of EGFR expression in the seven tumor types tested. Cetuximab was used as the primary mAb. b Immunofluorescence staining of EGFR-positive PC-3 and EGFR-negative MDA-MB-435 tumor tissues. Images were obtained under the same conditions and displayed at the same magnification and scale (200×)
a Correlation of EGFR expression level (measured by western blot) and the %ID/g values of seven different tumor types at 48 h p.i. (n = 3 per tumor model; n = 1 for MDA-MB-435). b Correlation of EGFR expression level (measured by western blot) and the tumor-to-muscle ratios of seven different tumor types at 48 h p.i. (n = 3 per tumor model; n = 1 for MDA-MB-435)
Human dosimetry extrapolation
For clinical translation of 64Cu-DOTA-cetuximab, human dosimetry estimation is important. The estimated absorbed doses for major organs and the whole body from microPET data in Sprague-Dawley rats are presented in Table 1. The tracer uptake of different organs in rats was similar to that in the mice except that the liver uptake in the rats appeared to be more prominent and persistent (Fig. 3c). Except for the blood, liver, and spleen, all organs exhibited a very low level of tracer uptake at all time points examined. There is no significant kidney uptake or renal excretion of the mAb-based tracer owing to the high molecular weight. These results predict that the highest radiation absorbed doses will be to the liver (0.41 ± 0.02 mGy/MBq). The whole-body absorbed dose was found to be 0.037 ± 0.001 mGy/MBq administered. Although the radiation doses to the liver and spleen were slightly higher than those for other 64Cu-labeled mAb reported in the literature [31], the radiation doses to the other major organs (kidneys, lungs, intestines, etc.) were much lower.
Discussion
Although cetuximab is already in the clinic and multiple advanced stage clinical trials [9–12], there has been no quantitative PET imaging of EGFR expression reported using radiolabeled cetuximab. In this study we successfully demonstrated that 64Cu-labeled cetuximab exhibits high receptor specificity both in vitro and in vivo. Most importantly, there is a good linear correlation (R 2 = 0.80) between the tumor uptake (measured by PET scans) and the EGFR expression level in vivo (measured by western blotting).
Non-invasive molecular imaging can give whole-body readout in live animals; help to decrease the workload and number of animals used; provide more statistically relevant results than in vitro studies as longitudinal studies can be performed in the same animal which can serve as its own control; aid in lesion detection in cancer patients and patient stratification; and help in individualized anticancer treatment monitoring and dose optimization [32]. Through the development of a robust imaging platform, molecular imaging can dramatically facilitate and speed up many steps of anticancer drug development in both the preclinical and clinical stages. The future of cancer therapy, molecular/personalized medicine, can only be achieved through quantitative molecular imaging. PET is a quantitative imaging modality and the agreement between the tumor/organ uptake measured by invasive biodistribution studies and the non-invasive PET scans has been vigorously validated [22, 24, 33]. Quantitative PET imaging of molecular cancer marker, such as EGFR, expression in vivo is critical in early and sensitive lesion detection, patient selection for entering clinical trials, better treatment monitoring and dose optimization based on non-invasive detection of early response to anticancer treatment, and elucidation of the mechanisms of treatment efficacy underlying the relevant signaling pathways.
Owing to the relatively high molecular weight of the mAb (150 kDa), 64Cu-DOTA-cetuximab clears from the body mainly through the hepatic route. The serum half-lives of 64Cu-DOTA-cetuximab in both mice and rats are less than 18 h based on microPET studies, much shorter than that in human [12], presumably because of the much faster metabolic rate of murine species as compared with human. Dosimetry of 99mTc-labeled cetuximab has been reported in cancer patients [13]. Although mAb-based tracer mainly excretes through the hepatic pathway, the highest radiation dose of 99mTc-cetuximab is to the kidney rather than the liver, indicating low stability of the 99mTc-labeled immunoconjugate. In this study, no kidney uptake was observed even at late time points (48 h), indicating that the 64Cu-DOTA complex is quite stable in vivo. The OLINDA software, as a replacement for MIRDOSE3, provides a calculation of the effective dose for human adults as defined in ICRP publication 60 [34]. The use of rodent biodistribution data is generally thought to give a worst-scenario estimate of absorbed doses to normal organs; whether this is true for 64Cu-DOTA-cetuximab remains to be tested in human patients. Nevertheless, for imaging purposes, the administered radiation dose is unlikely to cause any adverse effects since the amount injected is usually quite low (about 400 MBq) [35].
The half-life of 64Cu is 12.7 h. In this study we followed the tracer uptake for 48 h (about four half-lives of 64Cu). Longer term monitoring may be necessary for future studies and 89Zr (t 1/2 79.3 h) or 124I (t 1/2 100.8 h) may be used. It has been reported that 89Zr-labeled cetuximab reflects the biodistribution of 90Y-labeled cetuximab [36]. The 1:1 chelator/mAb ratio gave a significantly higher immunoreactive fraction (about 95%) of the resulting 89Zr- or 90Y-labeled cetuximab because of less disturbance on the antigen binding domain compared with the about 20 DOTA residues per cetuximab in our study. However, the tracer uptake in the tumor was comparable (15–25%ID/g). Although reducing the number of DOTA per cetuximab may increase the radioimmunoreactive fraction, the specific activity of 64Cu-DOTA-cetuximab will also be lower, which will lead to lower tumor uptake values [22]. There is a fine balance between the immunoreactive fraction and specific activity/tumor uptake and this may require more investigation in future studies.
In reality, it may be difficult to determine whether a tumor is EGFR positive if the tracer uptake in the tumor is not very high. The enhanced permeability and retention (EPR) effect (due to the leaky vasculature and lack of lymphatic drain in the tumor) may cause a certain level of tracer uptake in the tumor [37]. Tumor uptake solely attributed to the EPR effect has a characteristic pattern of reaching a maximum at early time points (the actual time differs between different tumors) followed by steady decrease afterwards. For specific targeting attributed to mAb–antigen interaction, the tumor uptake increases rapidly over time and reaches a plateau and remains steady, such as the U87MG and PC-3 tumor uptake observed in this study (Fig. 3b). Clinical translation of 64Cu-DOTA-cetuximab will be critical for the maximum benefit of cetuximab-based anticancer agents as imaging provides a straightforward and convenient way to monitor the biological changes at the molecular level in vivo. Multiple time point PET scans of the same patient over time may be required, and analysis of the tumor uptake pattern is suggested in order to differentiate EGFR specific activity accumulation from passive targeting due to the EPR effect.
Conclusion
We describe here the quantitative PET imaging of EGFR expression using 64Cu-labeled cetuximab, a chimeric mAb against EGFR. MicroPET imaging of 64Cu-DOTA-cetuximab showed prominent uptake in EGFR-expressing tumors but low accumulation in EGFR-negative tumors. Most importantly, there was a good linear correlation between the %ID/g values (measured by PET scans) and the EGFR expression level (measured by western blotting). The success of EGFR-specific tumor imaging using 64Cu-DOTA-cetuximab may be translated into the clinic. It may significantly expedite the process of clinical evaluation of cetuximab and provide maximum benefit in treating various types of EGFR-expressing cancer.
References
Arteaga C. Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol 2003;30:3–14.
Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 2005;6:32–43.
Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211–25.
Starling N, Cunningham D. Monoclonal antibodies against vascular endothelial growth factor and epidermal growth factor receptor in advanced colorectal cancers: present and future directions. Curr Opin Oncol 2004;16:385–90.
Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005;23:1137–46.
Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005;23:1126–36.
Ciardiello F, Damiano V, Bianco R, Bianco C, Fontanini G, De Laurentiis M, et al. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst 1996;88:1770–6.
Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003;21:2787–99.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78.
Thienelt CD, Bunn PA Jr, Hanna N, Rosenberg A, Needle MN, Long ME, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J Clin Oncol 2005;23:8786–93.
Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 2004;22:2610–6.
Frieze DA, McCune JS. Current status of cetuximab for the treatment of patients with solid tumors. Ann Pharmacother 2006;40:241–50.
Schechter NR, Wendt RE 3rd, Yang DJ, Azhdarinia A, Erwin WD, Stachowiak AM, et al. Radiation dosimetry of 99mTc-labeled C225 in patients with squamous cell carcinoma of the head and neck. J Nucl Med 2004;45:1683–7.
Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR, et al. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 2005;46:1898–906.
Velikyan I, Sundberg AL, Lindhe O, Hoglund AU, Eriksson O, Werner E, et al. Preparation and evaluation of 68Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med 2005;46:1881–8.
Wu AM, Williams LE, Zieran L, Padma A, Sherman MA, Bebb GG, et al. Anti-carcinoembryonic antigen (CEA) diabody for rapid tumor targeting and imaging. Tumor Targeting 1999;4:47–58.
Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, et al. MicroPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med 2004;45:1390–7.
Zhang X, Xiong Z, Wu X, Cai W, Tseng JR, Gambhir SS, et al. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med 2006;47:113–21.
Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 2006;6:669–76.
Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide (18F-FBEM), and the synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J Nucl Med 2006;47:1172–80.
Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, et al. MicroPET imaging of glioma αv-integrin expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med 2005;46:1707–18.
Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin™, a humanized monoclonal antibody against integrin αvβ3. Cancer Res 2006;66:9673–81.
Meares CF, McCall MJ, Reardan DT, Goodwin DA, Diamanti CI, McTigue M. Conjugation of antibodies with bifunctional chelating agents: isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal Biochem 1984;142:68–78.
Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–56.
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77–89.
Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, et al. Integrin αvβ3-targeted imaging of lung cancer. Neoplasia 2005;7:271–9.
Xiong Z, Cheng Z, Zhang X, Patel M, Wu JC, Gambhir SS, et al. Imaging chemically modified adenovirus for targeting tumors expressing integrin αvβ3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J Nucl Med 2006;47:130–9.
Visvikis D, Cheze-LeRest C, Costa DC, Bomanji J, Gacinovic S, Ell PJ. Influence of OSEM and segmented attenuation correction in the calculation of standardised uptake values for [18F]FDG PET. Eur J Nucl Med 2001;28:1326–35.
Anderson CJ, Jones LA, Bass LA, Sherman EL, McCarthy DW, Cutler PD, et al. Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor-bearing rats. J Nucl Med 1998;39:1944–51.
Sgouros G. Dosimetry of internal emitters. J Nucl Med 2005;46 Suppl 1:18S–27S.
Cutler PD, Schwarz SW, Anderson CJ, Connett JM, Welch MJ, Philpott GW, et al. Dosimetry of copper-64-labeled monoclonal antibody 1A3 as determined by PET imaging of the torso. J Nucl Med 1995;36:2363–71.
Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up anti-angiogenic drug development. Mol Cancer Ther 2006;5:2624–33.
Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic diabody. J Nucl Med 2007:in press.
International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection: ICRP publication 60. Ann ICRP 1991;21:6–10.
Philpott GW, Schwarz SW, Anderson CJ, Dehdashti F, Connett JM, Zinn KR, et al. RadioimmunoPET: detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J Nucl Med 1995;36:1818–24.
Verel I, Visser GW, Boellaard R, Boerman OC, van Eerd J, Snow GB, et al. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J Nucl Med 2003;44:1663–70.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics. A review. J Control Release 2000;65:271–84.
Acknowledgements
This project was financially supported by National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R21 EB001785), National Cancer Institute (NCI) (R21 CA102123, P50 CA114747, U54 CA119367, and R24 CA93862), Department of Defense (DOD) (W81XWH-04-1-0697, W81XWH-06-1-0665, W81XWH-06-1-0042, and DAMD17-03-1-0143), and a Benedict Cassen Postdoctoral Fellowship from the Education and Research Foundation of the Society of Nuclear Medicine (to W.Cai).
Author information
Authors and Affiliations
Corresponding author
Additional information
Weibo Cai and Kai Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cai, W., Chen, K., He, L. et al. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging 34, 850–858 (2007). https://doi.org/10.1007/s00259-006-0361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0361-6