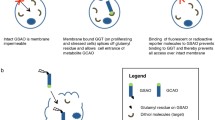
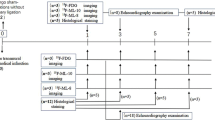
Abstract
Myocardial necrosis plays an important role in the pathogenesis of various cardiovascular disorders and can result from different myocardial insults. Its non-invasive identification and localisation therefore may help in the diagnosis of these disorders, as well as in prognosis and assessment of treatment response. Apoptosis, or programmed cell death, is important in the spectrum of myocardial damage since it is gradually becoming more apparent that cell death may begin as apoptosis and not as necrosis. First attempts to directly visualise the area of myocardial necrosis were based on recognition of myocardial infarction with "hot spot imaging agents" in patients with chest pain. Since then, the study of myocardial necrosis with gamma imaging agents has gone beyond the detection of myocardial infarction, and attempts have been made to diagnose other cardiovascular disorders associated with cardiac cell death such as heart transplant rejection, myocarditis, cardiotoxicity and cardiomyopathies. Traditionally, two hot spot imaging agents have been used for the detection of myocardial necrosis, 99mTc-pyrophosphate and 111In-antimyosin. In addition, preliminary studies have demonstrated promising results with 99mTc-glucarate. Recently, 99mTc-annexin V has been successfully used for non-invasive gamma imaging of apoptosis after acute myocardial infarction, acute myocardial ischaemia, acute cardiac allograft rejection and malignant intracardiac tumours. This review article focusses on the characteristics of these different myocardial necrotic and apoptotic markers and compares their role in the assessment of myocardial damage.


Similar content being viewed by others
References
Narula J, Zaret BL. Noninvasive detection of cell death: from tracking epitaphs to counting coffins. J Nucl Cardiol 2002; 9:554–560.
Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000; 342:1163–1170.
Rude RE, Poole WK, Muller JE, et al. Electrocardiographic and clinical criteria for recognition of acute myocardial infarction based on analysis of 3,697 patients. Am J Cardiol 1983; 52:936–942.
Holman BL, Lesch M, Zweiman FG. Detection and sizing of acute myocardial infarcts with99mTc(SN)tetracycline. N Engl J Med 1974; 291:159–163.
Bonte FJ, Parkey RW, Graham KD, Mohr J, Stokely EM. A new method for radionuclide imaging of myocardial infarcts. Radiology 1974; 110:473–474.
Beller GA, Khaw BA, Haber E, Smith TW. Localization of radiolabeled cardiac myosin-specific antibody in myocardial infarcts. Circulation 1977; 55:74–78.
Orlandi C, Crane PD, Edwards S, et al. Early scintigraphic detection of experimental myocardial infarction in dogs with technetium-99m-glucaric acid. J Nucl Med 1991; 32:236–238.
Khaw BA. The current role of infarct avid imaging. Semin Nucl Med 1999; 29:259–270.
Rude R, Parkey RW, Bonte FJ, et al. Clinical implication of the "doughnut" pattern of uptake in myocardial imaging with technetium-99 stannous pyrophosphate. Circulation 1977; 56:m-561.
Corbett JR, Lewis M, Willerson JT, et al. 99m-Tc-pyrophosphate imaging in patients with acute myocardial infarction: comparison of planar imaging with single-photon tomography with and without blood pool overlay. Circulation 1984; 69:1120–1128.
Massie BM, Botvinick EH, Werner JA, et al. Myocardial scintigraphy with technetium-99m stannous pyrophosphate: an insensitive test for nontransmural myocardial infarction. Am J Cardiol 1979; 43:186–192.
Khaw BA, Gold HK, Fallon JT, et al. Scintigraphic quantification of myocardial necrosis in patients after intravenous injection of cardiac myosin specific antibody. Circulation 1986; 74:501–508.
Khaw BA, Yasuda T, Gold HK, et al. Acute myocardial infarction imaging with indium-111 labeled monoclonal antimyosin Fab fragments. J Nucl Med 1987; 28:1671–1678.
Khaw BA, Gold HK, Leinbach RC, et al. Early imaging of experimental myocardial infarction by intracoronary administration of131I-labeled anticardiac myosin (Fab')2 fragments. Circulation 1978; 58:1137–1142.
Khaw BA, Fallon JT, Beller GA, Haber E. Specificity of localization of myosin-specific antibody fragments in experimental myocardial infarction: histologic, histochemical, autoradiographic and scintigraphic studies. Circulation 1979; 60:1527–1531.
Khaw BA, Scott J, Fallon JT, et al. Myocardial injury: quantitation by cell sorting initiated with antimyosin fluorescent spheres. Science 1982; 217:1050–1053.
Khaw BA, Mattis JA, Melincoff G, et al. Monoclonal antibody to cardiac myosin: imaging of experimental myocardial infarction. Hybridoma 1984; 3:11–23.
Narula J, Southern JF, Dec GW, et al. Antimyosin uptake and myofibrillarlysis in dilated cardiomyopathy. J Nucl Cardiol 1995; 2:470–477.
Carrió I, Bernà L, Ballester M, et al. Indium-111 antimyosin scintigraphy to assess myocardial damage in patients with suspected myocarditis and cardiac rejection. J Nucl Med 1988; 29:1893–1900.
Carrió I, López-Pousa A, Estorch M, et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J Nucl Med 1993; 34:1503–1507.
Nishimura T, Nagata S, Uehara T, et al. Assessment of myocardial damage in dilated-phase hypertrophic cardiomyopathy by using indium-111-antimyosin Fab myocardial scintigraphy. J Nucl Med 1991; 32:1333–1337.
Johnson LL, Seldin DW, Becker LC, et al. Antimyosin imaging in acute transmural myocardial infarction: results of a multicenter clinical trial. J Am Coll Cardiol 1989; 13:27–35.
Nedelman MA, Shealy DK, Boulin R, et al. Rapid infarct imaging with a technetium-99m-labeled antimyosin recombinant single chain Fv: evaluation in a canine model of acute myocardial infarction. J Nucl Med 1993; 34:234–241.
Tamaki N, Yamada T, Matsumori A, et al. Indium-111 antimyosin monoclonal antibody imaging for detecting different stages of myocardial infarction: comparison with technetium-99m-pyrophosphate imaging. J Nucl Med 1990; 31:136–142.
Frist W, Yasuda T, Segall G, et al. Noninvasive detection of human cardiac transplant rejection with In-111 antimyosin (Fab) imaging. Circulation 1987; 76 (Suppl V): 81–85.
Ballester M, Obrador D, Carrió I, et al.111In-monoclonal antimyosin antibody studies after the first year of heart transplantation: identification of risk groups for developing rejection during long-term follow-up and clinical implications. Circulation 1990; 82:2100–2108.
Ballester M, Obrador D, Carrió I, et al. Early postoperative reduction of monoclonal antimyosin antibody uptake is associated with absent rejection-related complications after heart transplantation. Circulation 1992; 85:61–68.
Hosenpud JD. Noninvasive diagnosis of cardiac allograft rejection. Another of many searches for the grail. Circulation 1992; 85:368–371.
Ballester M, Bordes R, Tazelaar T, et al. Evaluation of biopsy classification for rejection: relation to the detection of myocardial damage by 111-In-monoclonal antimyosin antibody imaging. J Am Coll Cardiol 1998; 31:1357–1361.
Estorch M, Carrió I, Berna L, et al.111In-antimyosin scintigraphy after doxorubicin therapy in patients with advanced breast cancer. J Nucl Med 1990; 31:1965–1969.
Carrió I, Estorch M, Bernà L, et al. Assessment of anthracycline induced myocardial damage by quantitative111In-antimyosin monoclonal antibody studies. Eur J Nucl Med 1991; 18:806–812.
Valdés Olmos RA, Carrió I, Hoefnagel M, et al. High sensitivity of radiolabelled antimyosin scintigraphy in assessing anthracycline related early myocyte damage preceding cardiac dysfunction. Nucl Med Commun 2002; 23:871–877.
Yasuda T, Palacios IF, Dec GW, et al. Indium-111 antimyosin monoclonal antibody imaging in the diagnosis of acute myocarditis. Circulation 1987; 76:306–311.
Dec GW, Palacios IF, Yasuda T, et al. Antimyosin antibody cardiac imaging: its role in the diagnosis of myocarditis. J Am Coll Cardiol 1990; 16:97–104.
Narula J, Khaw BA, Dec GW, et al. Recognition of acute myocarditis masquerading as acute myocardial infarction. N Engl J Med 1992; 328:100–104.
Obrador D, Ballester M, Carrió I, et al. High prevalence of myocardial monoclonal antimyosin antibody uptake in patients with chronic idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1989; 13:1289–1293.
Obrador D, Ballester M, Carrió I, et al. Active myocardial damage without attending inflammatory response in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1993; 21:1667–1671.
Obrador D, Ballester M, Carrió I, et al. Presence, evolving changes, and prognostic implications of myocardial damage detected in idiopathic and alcoholic dilated cardiomyopathy by111In monoclonal antimyosin antibodies. Circulation 1994; 89:2054–2061.
Pons-Llado G, Ballester M, Borras X, et al. Myocardial cell damage in human hypertension. J Am Coll Cardiol 2000; 36:2198–2203.
Van Vlies B, Van Royen EA, Visser CA, et al. Frequency of myocardial indium-111 antimyosin uptake alter uncomplicated coronary artery bypass grafting. Am J Cardiol 1990; 66:1191–1195.
Narula J. Pathologic basis for the role of antimyosin imaging for the detection of cardiac involvement in systemic disorders. In: Khaw BA, Narula J, Strauss WH, eds. Monoclonal antibodies in cardiovascular disease. Phildelphia: Lea and Febiger; 1994:118–126.
Martí V, Ballester M, Udina C, et al. Evaluation of myocardial cell damage by In-111 monoclonal antimyosin antibodies in patients under chronic tricyclic antidepressant drug treatment. Circulation 1995; 92:1619–1623.
Martí V, Ballester M, Obrador D, et al. Active myocardial damage in hyperthyroidism: a concurrent mechanism of heart failure reversed by treatment. Eur Heart J 1995; 16:1014–1016.
Martí V, Ballester M, Rigla M, et al. Myocardial damage does not occur in untreated hyperthyroidism unless associated with congestive heart failure. Am Heart J 1997; 134:1133–1137.
Ewer MS, Ali MK, Mackey B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adryamicin. J Clin Oncol 1984; 2:112–117.
Sarda L, Colin P, Boccara F, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001; 37:786–792.
Martí V, Aymat R, Ballester M, García J, Carrió I, Augé JM. Coronary endothelial dysfunction and myocardial cell damage in chronic stable idiopathic dilated cardiomyopathy. Int J Cardiol 2002; 82:237–245.
Flotats A, Domingo P, Carrió I. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med 1999; 340:733–734.
Data on file. Centocor, Inc. Myoscint PLA 95–1210.
Mariani G, Villa G, Rossettin PF, et al. Detection of myocardial infarction by99mTc-labeled d-glucaric acid imaging in patients with acute chest pain. J Nucl Med 1999; 40:1832–1839.
Narula J, Petrov A, Pak KY, et al. Very early noninvasive detection of acute experimental nonreperfused myocardial infarction with99mTc-labeled glucarate. Circulation 1995; 95:1577–1584.
Khaw BA, Nakazama A, O'Donell SM, et al. Avidity of technetium 99m glucarate for the necrotic myocardium: in vivo and in vitro assessment. J Nucl Cardiol 1997; 4:283–290.
Fornet BB, Yasuda T, Wilkinson R, et al. Detection of acute cardiac injury with technetium-99m glucaric acid [abstract]. J Nucl Med 1989; 30:1743.
Yaoita H, Fischman AJ, Wilkinson R, et al. Distribution of deoxyglucose and technetium-99m-glucarate in the acutely ischemic myocardium. J Nucl Med 1993; 34:1303–1308.
Ohtani H, Callahan RJ, Khaw BA, et al. Comparison of technetium-99m-glucarate and thallium-201 for the identification of acute myocardial infarction in rats. J Nucl Med 1992; 33:1988–1993.
Beanlands RSB, Ruddy TD, Bielawsky L, et al. Differentiation of myocardial ischemia and necrosis by technetium 99m glucaric acid kinetics. J Nucl Cardiol 1997; 4:274–282.
Johnson LL, Schofield L, Mastrofrancesco P, Donahay T, Farb A, Khaw BA. Tc-99m glucarate uptake in a swine model of limited flow plus increased demand. J Nucl Cardiol 2000; 7:579–588.
Khaw BA, Da Silva J, Petrov A, Hartner W. Indium 111 antimyosin and Tc-99m glucaric acid for noninvasive identification of oncotic and apoptotic necrosis. J Nucl Cardiol 2002; 9:471–481.
Puig M, Ballester M, Matías-Guiu X, et al. Burden of myocardial damage in cardiac allograft rejection: scintigraphic evidence of uptake of myocardial injury and histologic evidence of myocyte necrosis and apoptosis. J Nucl Cardiol 2000; 7:132–139.
Blankenberg FG, Katsikis PD, Tait JF, et al. Comparison of Tc-99m glucarate uptake in apoptotic (programmed) and necrotic cell death [abstract]. J Nucl Med 1997; 38:192P.
Molea N, Lazzeri E, Bodei L, et al. Biodistribution pharmacokinetics and dosimetry of99mTc-d-glucaric acid in humans. In: Bergmann H, Kroiss A, Sinzinger H, eds. Radioactive isotopes in clinical medicine and research. XXI. Basel: Birkhauser; 1997:359–364.
Flotats A, Narula J, Santaló M, et al. Myocardial uptake of technetium-99m glucarate occurs in acute regional myocardial necrosis but not in diffuse ongoing myocardial damage [abstract]. J Nucl Cardiol 1999; 6 (Suppl):S100.
Gerson MC, McGoron AJ. Technetium 99m-glucarate: what will be its clinical role? J Nucl Cardiol 1997; 4:336–340.
Blankenberg F, Narula J, Strauss HW. In vivo detection of apoptotic cell death: a necessary measurement for evaluating therapy for myocaditis, ischemia, and heart failure. J Nucl Cardiol 1999; 6:531–539.
Holly TA, Drincic A, Byaun Y, et al. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol 1999; 31:1709–1715.
Green DR. Apoptotic pathways: the roads to ruin. Cell 1998; 94:695–698.
Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell 2000; 102:1–4.
van Heerde WL, Robert-Offerman S, Dumont E, et al. Markers of apoptosis in cardiovascular tissues: focus on annexin V. Cardiovasc Res 2000; 45:549–559.
Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997; 89:1121–1132.
Martin SJ, Reutelingsperger CPM, McGahon AJ. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182:1545–1556.
Fadok VA, Bratton DL, Rose DM, et al. A receptor for phosphatidylserine specific clearance of apoptotic cells. Nature 2000; 405:85–90.
Strauss HW, Narula J, Blankenberg FG. Radioimaging to identify myocardial cell death and probably injury. Lancet 2000; 356:180–181.
Romisch J, Paques EP. Annexins: calcium binding proteins of multifunctional importance? Med Microbiol Immunol 1991; 180:109–126.
van Heerde WL, de Groot PG, Reutelingsperger CPM. The complexity of the phospholipid binding protein annexin V. Thromb Haemost 1995; 73:172–179.
Tait JF, Cerqueira MD, Dewhurst TA, Fujikawa K, Ritchie JL, Stratton JR. Evaluation of annexin V as a platelet directed thrombus targeting agent. Thromb Res 1994; 75:491–501.
Stratton JR, Dewhurst TA, Kasina S, et al. Selective uptake of radiolabeled annexin V on acute porcine left atrial thrombi. Circulation 1995; 92:3113–3121.
Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, vanOers MHJ. Annexin V for cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84:1415–1420.
Boersma AMW, Kees N, Oostrum RG, Stoter RG. Quantification of apoptotic cells with fluorescein isothiocynate-labeled annexin V in Chinese hamster ovary cell cultures treated with cisplatin. Cytometry 1996; 24:123–130.
Bennett MR, Gibson DF, Schwart S, Tait JF. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res 1995; 77:1136–1142.
Tait JF, Smith C, Wood BL. Measurement of phosphatidylserine exposure in leukocytes and platelets by whole-blood flow cytometry with annexin V. Blood Cells Mol Dis 1999; 25:271–278.
Dischman AJ, Khaw BA, Strauss HW. Quo vadis radioimmune imaging. J Nucl Med 1999; 30:1911–1915.
Gidon-Jeangirard C, Solito E, Hofmann A, et al. Annexin V counteracts apoptosis while inducing Ca2+ influx in human lymphocytic T cells. Biochem Biophys Res Commun 1999; 265:709–715.
Kaneko N, Matsuda R, Hosoda S, et al. Measurement of plasma annexin V by ELISA in the early detection of acute myocardial infarction. Clin Chim Acta 1996; 251:65–80.
van den Eijnde SM, Luijsterburg AJ, Boshart L, et al. In situ detection of apoptosis during embryogenesis with annexin V: from whole mount to ultrastructure. Cytometry 1997; 29:313–320.
Dumont EA, Hofstra L, van Heerde WL, et al. Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human annexin-V in a mouse model. Circulation 2000; 102:1564–1568.
Dumont EA, Reutelingsperger CP, Smits JF, et al. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med 2001; 7:1352–1355.
Blankenberg FG, Katsikis PD, Tait JF, et al. In vivo detection and imaging of phosphtidylserine expression during programmed cell death. Proc Natl Acad Sci U S A 1998; 95:6349–6354.
Wood BL, Gibson DF, Tait JF. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood 1996; 88:1873–1880.
Tait JF, Engelhardt S, Smith C, Fujikawa K. Pro-urokinase-annexin V chimeras. Construction, expression, and characterization of recombinant proteins. J Biol Chem 1995; 270:21594–21599.
Tait JF, Smith C. Site-specific mutagenesis of annexin V: role of residues from Arg-200 to Lys-207 in phospholipids binding. Arch Biochem Biophys 1991; 288:141–144.
Abrams MJ, Juweid M, tenKate CI, et al. Technetium-99m-human polyclonal IgG radiloabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med 1990; 31:2022–2028.
Larsen SK, Solomon HF, Caldwell G, Abrams MJ. [99mTc]tricine: a useful precursor complex for the radiolabeling of hydrazinonicotinate protein conjugates. Bioconjug Chem 1995; 6:635–638.
Blankenberg FG, Katsikis PD, Tait JF, et al. Imaging of apoptosis (programmed cell death) with99mTc annexin V. J Nucl Med 1999; 40:184–191.
Ohtsuki K, Akashi K, Aoka Y, et al.99mTc-HYNIC annexin V: a radiopharmaceutical for the in vivo detection of apoptosis. Eur J Nucl Med 1999; 26:1251–1258.
Vriens PW, Blankenberg FG, Stoot JH, et al. The use of technetium Tc99m annexin V for in vivo imaging of apoptosis during cardiac allograft rejection. J Thorac Cardiovasc Surg 1998; 116:844–853.
Ogura Y, Krams SM, Martinez OM, et al. Radiolabeled annexin V imaging: diagnosis of allograft rejection in an experimental rodent model of liver transplantation. Radiology 2000; 214:795–800.
Blankenberg FG, Robbins RC, Stoot JH, et al. Radionuclide imaging of acute lung transplant rejection with annexin V. Chest 2000; 117:834–840.
D'Arceuil HE, Rhine W, de Crespigny A, et al.99mTc Annexin V imaging of neonatal hypoxic brain injury. Stroke 2000; 32:2692–2700.
Blankenberg FG, Naumovski K, Tait JF, Post AM, Strauss HW. Imaging cyclophosphamide-induced intramedullary apoptosis in rats using99mTc-radiolabeled annexin. J Nucl Med 2001; 42:309–316.
Post AM, Katsikis PD, Tait JF, Geaghan SM, Strauss HW, Blankenberg FG. Imaging cell death with radiolabeled annexin V in an experimental model of rheumatoid arthritis. J Nucl Med 2002; 43:1359–1365.
Tokita N, Hasegawa S, Maruyama K, et al.99mTc-Hynic-annexin V imaging to evaluate inflammation and apoptosis in rats with autoimmune myocarditis. Eur J Nucl Med 2003; 30:232–238.
Kemerink GJ, Liem IH, Hofstra L, et al. Patient dosimetry of intravenously administered99mTc-annexin V. J Nucl Med 2001; 42:382–387.
Kemerink GJ, Boersma HH, Thimister PWL, et al. Biodistribution and dosimetry of99mTc-BTAP-annexin-V. Eur J Nucl Med 2001; 28:1373–1378.
Hofstra L, Liem IH, Dumont EA, et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet 2000; 356:209–212.
Narula J, Acio ER, Narula N, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med 2001; 7:1347–1352.
Kown HM, Strauss HM, Blankenberg FG, et al. In vivo imaging of acute cardiac rejection in human patients using (99m)technetium labeled annexin. Am J Transplant 2001; 1:270–277.
Myocardial Infarction Redefined—A Consensus Document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol 2000; 3:959–969.
Li C, Wen X, Wu Q, et al. Apoptosis induced by drug treatments correlates with uptake of 111-In-labeled PEGylated annexin V in MDA-MB468 tumors. J Nucl Med 2002; 43 (Suppl):41P.
Russell J, O'Donoghue JA, Finn R, et al. Iodination of annexin V for imaging apoptosis. J Nucl Med 2002; 43:671–677.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flotats, A., Carrió, I. Non-invasive in vivo imaging of myocardial apoptosis and necrosis. Eur J Nucl Med Mol Imaging 30, 615–630 (2003). https://doi.org/10.1007/s00259-003-1136-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1136-y