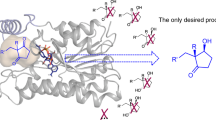
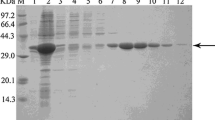
Abstract
Ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE) is an important chiral intermediate for the synthesis of “blockbuster” drug statins. The carbonyl reductase ChKRED20 from Chryseobacterium sp. CA49 was found to catalyze the bio-reductive production of (S)-CHBE with excellent stereoselectivity (>99.5 % ee). Perceiving a capacity for improvement, we sought to increase the thermostability of ChKRED20 to allow a higher reaction temperature. After one round of error-prone PCR (epPCR) library screening followed by the combination of beneficial mutations, a triple-mutant MC135 was successfully achieved with substantially enhanced thermostablity. The activity of MC135 at 50 °C was similar to the wild type. However, at its temperature optima of 65 °C, the mutant displayed 63 % increase of activity compared to the wild type and remained >95 % activity after being incubated for 15 days, while the wild type had a half-life of 11.9 min at 65 °C. At a substrate/catalyst ratio of 100 (w/w), the mutant catalyzed the complete conversion of 300 g/l substrate within 1 h to yield enantiopure (S)-CHBE with an isolated yield of 95 %, corresponding to a space-time yield of 1824 mM/h.






Similar content being viewed by others
References
Babu MM (2003) NCI: a server to identify non-canonical interactions in protein structures. Nucleic Acids Res 31(13):3345–3348
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(W1):W252–W258
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485(7397):185–194
Chen S-Y, Yang C-X, Wu J-P, Xu G, Yang L-R (2013) Multi-enzymatic biosynthesis of chiral β-hydroxy nitriles through co-expression of oxidoreductase and halohydrin dehalogenase. Adv Synth Catal 355(16):3179–3190
Giver L, Gershenson A, Freskgard P-O, Arnold FH (1998) Directed evolution of a thermostable esterase. Proc Natl Acad Sci U S A 95:12809–12813
Goldberg K, Schroer K, Lutz S, Liese A (2007) Biocatalytic ketone reduction-a powerful tool for the production of chiral alcohols-part I: processes with isolated enzymes. Appl Microbiol Biotechnol 76(2):237–248
Gooding OW, Voladr R, Bautista A, Hopkins T (2010) Development of a practical biocatalytic process for (R)-2-methylpentanol. Org Process Res Dev 14(1):119–126
Hirokawa K, Ichiyanagi A, Kajiyama N (2008) Enhancement of thermostability of fungal deglycating enzymes by directed evolution. Appl Microbiol Biotechnol 78:775–781
Hollmann F, Arends IWCE, Holtmann D (2011) Enzymatic reductions for the chemist. Green Chem 13:2285–2314
Huisman GW, Liang J, Krebber A (2010) Practical chiral alcohol manufacture using ketoreductases. Curr Opin Chem Biol 14(2):122–129
Jung J, Park HJ, Uhm K-N, Kim D, Kim H-K (2010) Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxy butanoate using a Saccharomyces cerevisiae reductase: enantioselectivity and enzyme–substrate docking studies. Biochim Biophys Acta 1804(9):1841–1849
Kaluzna IA, Feske BD, Wittayanan W, Ghiviriga I, Stewart JD (2005) Stereoselective, biocatalytic reductions of α-chloro-β-keto esters. J Org Chem 70(1):342–345
Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL repository and associated resources. Nucleic Acids Res 37:D387–D392
Kita K, Fukura T, Nakase K, Okamoto K, Yanase H, Kataoka M, Shimizu S (1999) Cloning, overexpression, and mutagenesis of the Sporobolomyces salmonicolor AKU4429 gene encoding a new aldehyde reductase, which catalyzes the stereoselective reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (S)-4-chloro-3-hydroxybutanoate. Appl Environ Microbiol 65(12):5207–5211
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55(5):590–595
Kosjek B, Nti-Gyabaah J, Telari K, Dunne L, Moore JC (2008) Preparative asymmetric synthesis of 4,4-dimethoxytetrahydro-2H-pyran-3-ol with a ketone reductase and in situ cofactor recycling using glucose dehydrogenase. Org Process Res Dev 12(4):584–588
Lee S-H, Park O-J (2009) Uses and production of chiral 3-hydroxy-gamma-butyrolactones and structurally related chemicals. Appl Microbiol Biotechnol 84(5):817–828
Liang J, Mundorff E, Voladri R, Jenne S, Gilson L, Conway A, Krebber A, Wong J, Huisman G, Truesdell S, Lalonde J (2010) Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol. Org Process Res Dev 14(1):188–192
Liu Y, Tang TX, Pei XQ, Zhang C, Wu ZL (2014) Identification of ketone reductase ChKRED20 from the genome of Chryseobacterium sp CA49 for highly efficient anti-Prelog reduction of 3,5-bis(trifluoromethyl)acetophenone. J Mol Catal B Enzym 102:1–8
Liu Z-Q, Ye J-J, Shen Z-Y, Hong H-B, Yan J-B, Lin Y, Chen Z-X, Zheng Y-G, Shen Y-C (2015) Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl Microbiol Biotechnol 99(5):2119–2129
Ma SK, Gruber J, Davis C, Newman L, Gray D, Wang A, Grate J, Huisman GW, Sheldon RA (2010) A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem 12(1):81–86
McLachlan MJ, Johannes TW, Zhao H (2008) Further improvement of phosphite dehydrogenase thermostability by saturation mutagenesis. Biotechnol Bioeng 99(2):268–274
Moore JC, Pollard DJ, Kosjek B, Devine PN (2007) Advances in the enzymatic reduction of ketones. Acc Chem Res 40(12):1412–1419
Ni Y, Xu JH (2012) Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols. Biotechnol Adv 30:1279–1288
Patel RN (2008) Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord Chem Rev 252:659–701
Pei XQ, Yi ZL, Tang CG, Wu ZL (2011) Three amino acid changes contribute markedly to the thermostability of beta-glucosidase BglC from Thermobifida fusca. Bioresour Technol 102(3):3337–3342
Pennacchio A, Sannino V, Sorrentino G, Rossi M, Raia CA, Esposito L (2013) Biochemical and structural characterization of recombinant short-chain NAD(H)-dependent dehydrogenase/reductase from Sulfolobus acidocaldarius highly enantioselective on diaryl diketone benzil. Appl Microbiol Biotechnol 97(9):3949–3964
Qiu L, Qi J, Pai C-C, Chan S, Zhou Z, Choi MCK, Chan ASC (2002) Synthesis of novel diastereomeric diphosphine ligands and their applications in asymmetric hydrogenation reactions. Org Lett 4(26):4599–4602
Rodrigues RC, Berenguer-Murcia A, Fernandez-Lafuente R (2011) Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv Synth Catal 353(13):2216–2238
Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torres R, Fernandez-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42(15):6290–6307
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphasic system. Appl Environ Microbiol 56(8):2374–2377
Solano DM, Hoyos P, Hernáiz MJ, Alcántara AR, Sánchez-Montero JM (2012) Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour Technol 115:196–207
Tang T-X, Liu Y, Wu Z-L (2014) Characterization of a robust anti-Prelog short-chain dehydrogenase/reductase ChKRED20 from Chryseobacterium sp. CA49. J Mol Catal B Enzym 105:82–88
Tina KG, Bhadra R, Srinivasan N (2007) PIC: protein interactions calculator. Nucleic Acids Res 35:W473–W476
Vieille C, Gregory Zeikus J (1996) Thermozymes: Identifying molecular determinants of protein structural and functional stability. Trends Biotechnol 14(6):183–190
Vieira DS, Degrève L, Ward RJ (2009) Characterization of temperature dependent and substratebinding cleft movements in Bacillus circulans family 11 xylanase: A molecular dynamics investigation. Biochimica et Biophysica Acta (BBA) - General Subjects 1790(10):1301–1306
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269(4):631–643
Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8(1):52–56
Xu Z, Liu Y, Fang L, Jiang X, Jing K, Cen P (2006) Construction of a two-strain system for asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to (S)-4-chloro-3-hydroxybutanoate ethyl ester. Appl Microbiol Biotechnol 70(1):40–46
Ye Q, Ouyang P, Ying H (2011) Biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89(3):513–522
You ZY, Liu ZQ, Zheng YG (2014) Characterization of a newly synthesized carbonyl reductase and construction of a biocatalytic process for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with high space-time yield. Appl Microbiol Biotechnol 98(4):1671–1680
Zhang SB, Pei XQ, Wu ZL (2012) Multiple amino acid substitutions significantly improve the thermostability of feruloyl esterase A from Aspergillus niger. Bioresour Technol 117:140–147
Zhao H, van der Donk WA (2003) Regeneration of cofactors for use in biocatalysis. Curr Opin Biotechnol 14(6):583–589
Acknowledgments
We thank Professor Ganggang Wang and Mr. Yun Jin of the Chengdu Institute of Biology for the size-exclusion chromatography analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (21372216 and 21572220), the Open Fund of Key Laboratory of Environmental and Applied Microbiology (KLCAS-2014-05 and KLCAS-2015-01), and the Key Research Program (KGZD-EW-606-14) of the Chinese Academy of Sciences.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1696 kb)
Rights and permissions
About this article
Cite this article
Zhao, FJ., Pei, XQ., Ren, ZQ. et al. Rapid asymmetric reduction of ethyl 4-chloro-3-oxobutanoate using a thermostabilized mutant of ketoreductase ChKRED20. Appl Microbiol Biotechnol 100, 3567–3575 (2016). https://doi.org/10.1007/s00253-015-7200-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7200-2