Abstract
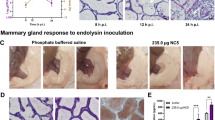
Bovine mastitis results in billion dollar losses annually in the USA alone. Streptococci are among the most relevant causative agents of this disease. Conventional antibiotic therapy is often unsuccessful and contributes to development of antibiotic resistance. Bacteriophage endolysins represent a new class of antimicrobials against these bacteria. In this work, we characterized the endolysins (lysins) of the streptococcal phages λSA2 and B30 and evaluated their potential as anti-mastitis agents. When tested in vitro against live streptococci, both enzymes exhibited near-optimum lytic activities at ionic strengths, pH, and Ca2+ concentrations consistent with cow milk. When tested in combination in a checkerboard assay, the lysins were found to exhibit strong synergy. The λSA2 lysin displayed high activity in milk against Streptococcus dysgalactiae (reduction of CFU/ml by 3.5 log units at 100 μg/ml), Streptococcus agalactiae (2 log), and Streptococcus uberis (4 log), whereas the B30 lysin was less effective. In a mouse model of bovine mastitis, both enzymes significantly reduced intramammary concentrations of all three streptococcal species (except for B30 vs. S. dysgalactiae), and the effects on mammary gland wet weights and TNFα concentrations were consistent with these findings. Unexpectedly, the synergistic effect determined for the two enzymes in vitro was not observed in the mouse model. Overall, our results illustrate the potential of endolysins for treatment of Streptococcus-induced bovine mastitis.




Similar content being viewed by others
References
Anderson JC, Craven N (1984) Assessment in the mouse of cefoperazone as a treatment for mastitis. Vet Rec 114:607–612
Apparao MD, Ruegg PL, Lago A, Godden S, Bey R, Leslie K (2009) Relationship between in vitro susceptibility test results and treatment outcomes for gram-positive mastitis pathogens following treatment with cephapirin sodium. J Dairy Sci 92:2589–2597. doi:10.3168/jds.2008-1693
Bateman A, Rawlings ND (2003) The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends BiochemSci 28:234–237
Becker SC, Foster-Frey J, Donovan DM (2008) The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett 287:185–191
Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM (2009) Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 443:32–41
Blowey RW, Edmondson P (2010) Mastitis control in dairy herds. CABI, Cambridge
Borysowski J, Weber-Dabrowska B, Gorski A (2006) Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med(Maywood) 231:366–377
Bramley AJ, Foster R (1990) Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res Vet Sci 49:120–121
Brouillette E, Malouin F (2005) The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect 7:560–568
Brouillette E, Grondin G, Lefebvre C, Talbot BG, Malouin F (2004) Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet Microbiol 101:253–262
Calvinho LF, Almeida RA, Oliver SP (1998) Potential virulence factors of Streptococcus dysgalactiae associated with bovine mastitis. Vet Microbiol 61:93–110
Cattell MB, Dinsmore RP, Belschner AP, Carmen J, Goodell G (2001) Environmental gram-positive mastitis treatment: in vitro sensitivity and bacteriologic cure. J Dairy Sci 84:2036–2043
Celia LK, Nelson D, Kerr DE (2008) Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet Microbiol 130:107–117
Chandler RL (1970a) Experimental bacterial mastitis in the mouse. J Med Microbiol 3:273–282
Chandler RL (1970b) Ultrastructural pathology of mastitis in the mouse. A study of experimental staphylococcal and streptococcal infections. Br J Exp Pathol 51:639–645
Chandler RL (1971) Studies on experimental mouse mastitis relative to the assessment of pharmaceutical substances. J Comp Pathol 81:507–514
Deluyker HA, Van Oye SN, Boucher JF (2005) Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows. J Dairy Sci 88:604–614
Demon D, Ludwig C, Breyne K, Guede D, Dorner JC, Froyman R, Meyer E (2012) The intramammary efficacy of first generation cephalosporins against Staphylococcus aureus mastitis in mice. Vet Microbiol 160:141–150. doi:10.1016/j.vetmic.2012.05.017
Demon D, Breyne K, Schiffer G, Meyer E (2013) Short communication: Antimicrobial efficacy of intramammary treatment with a novel biphenomycin compound against Staphylococcus aureus, Streptococcus uberis, and Escherichia coli-induced mouse mastitis. J Dairy Sci 96:7082–7087. doi:10.3168/jds.2013-7011
Diarra MS, Petitclerc D, Deschenes E, Lessard N, Grondin G, Talbot BG, Lacasse P (2003) Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet Immunol and Immunopathol 95:33–42
Donovan DM (2007) Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat Biotechnol 1:113–122
Donovan DM, Foster-Frey J (2008) LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol Lett 287:22–33
Donovan DM, Dong S, Garrett W, Rousseau GM, Moineau S, Pritchard DG (2006a) Peptidoglycan hydrolase fusions maintain their parental specificities. Appl Environ Microbiol 72:2988–2996
Donovan DM, Foster-Frey J, Dong S, Rousseau GM, Moineau S, Pritchard DG (2006b) The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl Environ Microbiol 72:5108–5112
Donovan DM, Lardeo M, Foster-Frey J (2006c) Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett 265:133–139
Donovan DM, Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG (2009) Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech International 21:6–10
Fischetti VA (2005) Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol 13:491–496
Gao J, Yu FQ, Luo LP, He JZ, Hou RG, Zhang HQ, Li SM, Su JL, Han B (2012) Antibiotic resistance of Streptococcus agalactiae from cows with mastitis. Vet J 194:423–424. doi:10.1016/j.tvjl.2012.04.020
Garcia P, Garcia JL, Garcia E, Sanchez-Puelles JM, Lopez R (1990) Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene 86:81–88
Gaucheron F (2005) The minerals of milk. Reprod Nutr Dev 45:473–483. doi:10.1051/rnd:2005030
Gehring R, Smith GW (2006) An overview of factors affecting the disposition of intramammary preparations used to treat bovine mastitis. J Vet Pharmacol Ther 29:237–241
Gilmer DB, Schmitz JE, Euler CW, Fischetti VA (2013) Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi:10.1128/AAC.02526-12
Gruet P, Maincent P, Berthelot X, Kaltsatos V (2001) Bovine mastitis and intramammary drug delivery: review and perspectives. Adv Drug Deliv Rev 50:245–259
Hermoso JA, Garcia JL, Garcia P (2007) Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461–472
Kerr DE, Plaut K, Bramley AJ, Williamson CM, Lax AJ, Moore K, Wells KD, Wall RJ (2001) Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat Biotechnol 19:66–70
Kuang Y, Jia H, Miyanaga K, Tanji Y (2009) Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl Microbiol Biotechnol 84:135–142
Lammers A, van Vorstenbosch CJ, Erkens JH, Smith HE (2001) The major bovine mastitis pathogens have different cell tropisms in cultures of bovine mammary gland cells. Vet Microbiol 80:255–265
Leigh JA (1999) Streptococcus uberis: a permanent barrier to the control of bovine mastitis? Vet J 157:225–238. doi:10.1053/tvjl.1998.0298
Lewis RE, Diekema DJ, Messer SA, Pfaller MA, Klepser ME (2002) Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J Antimicrob Chemother 49:345–351
Loeffler JM, Fischetti VA (2003) Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377
Loeffler JM, Nelson D, Fischetti VA (2001) Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172
Lonnerdal B, Keen CL, Hurley LS (1981) Iron, copper, zinc, and manganese in milk. Annu Rev Nutr 1:149–174. doi:10.1146/annurev.nu.01.070181.001053
Lopez R, Garcia E (2004) Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev 28:553–580
Notebaert S, Meyer E (2006) Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet Q 28:2–13
Obeso JM, Martinez B, Rodriguez A, Garcia P (2008) Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int J Food Microbiol 128:212–218
Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi:10.1093/jac/dkg301
O’Flaherty S, Coffey A, Meaney WJ, Fitzgerald GF, Ross RP (2005) Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett Appl Microbiol 41:274–279
Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA (2011) A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. doi:10.1128/AAC.00890-10
Ponting CP, Aravind L, Schultz J, Bork P, Koonin EV (1999) Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J Mol Biol 289:729–745
Pritchard DG, Dong S, Baker JR, Engler JA (2004) The bifunctional peptidoglycan lysin of Streptococcus agalactieae bacteriophage B30. Microbiology 150:2079–2087
Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR (2007) LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol 73:7150–7154
Pyörälä S (2009) Treatment of mastitis during lactation. Ir Vet J 62(Suppl 4):S40–S44. doi:10.1186/2046-0481-62-S4-S40
Reichelt P, Schwarz C, Donzeau M (2006) Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr Purif 46:483–488
Rowson AD, Wang YQ, Aalseth E, Forsberg NE, Puntenney SB (2011) Effects of an immunomodulatory feed additive on the development of mastitis in a mouse infection model using four bovine-origin isolates. Animal 5(2):220–229. doi:10.1017/S1751731110001850
Sanchez MS, Ford CW, Yancey RJ Jr (1994) Effect of tumor necrosis factor-alpha, interleukin-1 beta, and antibiotics on the killing of intracellular Staphylococcus aureus. J Dairy Sci 77:1251–1258
Schindler CA, Schuhardt VT (1964) Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc Natl Acad Sci U S A 51:414–421
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477
Schmelcher M, Shabarova T, Eugster MR, Eichenseher F, Tchang VS, Banz M, Loessner MJ (2010) Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol 76:5745–5756
Schmelcher M, Donovan DM, Loessner MJ (2012a) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. doi:10.2217/fmb.12.97
Schmelcher M, Powell AM, Becker SC, Camp MJ, Donovan DM (2012b) Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl Environ Microbiol 78:2297–2305. doi:10.1128/AEM.07050-11
Schuch R, Nelson D, Fischetti VA (2002) A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889
Sordillo LM, Streicher KL (2002) Mammary gland immunity and mastitis susceptibility. J Mammary Gland Biol Neoplasia 7:135–146
Tenhagen BA, Koster G, Wallmann J, Heuwieser W (2006) Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J Dairy Sci 89:2542–2551. doi:10.3168/jds.S0022-0302(06)72330-X
Wall RJ, Powell A, Paape MJ, Kerr DE, Bannerman DD, Pursel VG, Wells KD, Talbot N, Hawk HW (2005) Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol 23:445–451
Wall R, Powell A, Sohn E, Foster-Frey J, Bannerman D, Paape M (2009) Enhanced host immune recognition of mastitis causing Escherchia coli in CD-14 transgenic mice. Anim Biotechnol 20:1–14
Walsh C (2003) Where will new antibiotics come from? Nat Rev Microbiol 1:65–70
Webb BH, Johnson AH, Alford JA (1974) Fundamentals of dairy chemistry. AVI, Westport
Whisstock JC, Lesk AM (1999) SH3 domains in prokaryotes. Trends Biochem Sci 24:132–133
Wilson DJ, Gonzalez RN, Das HH (1997) Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J Dairy Sci 80:2592–2598
Yancey RJ Jr (1999) Vaccines and diagnostic methods for bovine mastitis: fact and fiction. Adv Vet Med 41:257–273
Acknowledgments
Mentioning of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. USDA is an equal opportunity provider and employer.
Funding
This work was supported in part by National Institutes of Health grant 1 R01 AI075077-01A1; National Research Initiative grant 2007-35204-18395 and US State Department funds supporting US-Pakistani and US-Russian collaborations.
Compliance with Ethical Standards
ᅟ
Conflict of interest
The authors declare that they have no conflict of interest.
Statement regarding research on animals
All animal experiments were conducted in accordance with ARS Institutional Animal Care and Use Committee regulations and national animal care guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmelcher, M., Powell, A.M., Camp, M.J. et al. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl Microbiol Biotechnol 99, 8475–8486 (2015). https://doi.org/10.1007/s00253-015-6579-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6579-0