Abstract
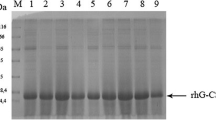
Since its isolation, the human granulocyte–macrophage colony-stimulating factor (hGM-CSF) has been proposed as a new class of therapeutic biological products in the treatment of various diseases. However, the toxicity of this cytokine towards its expression host constitutes a major obstacle to bioprocess development for large-scale production. In this work, the optimized gene encoding hGM-CSF was expressed in the yeast Yarrowia lipolytica in one and two copies under the control of the fatty acid-inducible POX2 promoter. Protein secretion was directed by the targeting sequence of the extracellular lipase (LIP2): preXALip2. After 48 h of induction, Western blot analysis revealed the presence of a nonglycosylated form of 14.5 kDa and a trail of hGM-CSF hyperglycosylated varying from 23 kDa to more than 60 kDa. The two-copy transformants produced hGM-CSF level which was sevenfold higher compared to the single-copy ones. Deglycosylation with PNGase F showed two forms: a mature form of 14.5 kDa and an unprocessed form of 18 kDa. The addition of two alanines to the signal sequence resulted in correct hGM-CSF processing. The production level was estimated at 250 mg/l after preliminary optimization studies of the cultivation and induction phases. The purified hGM-CSF was identified by N-terminal sequencing and LC-MS/MS analysis; its biological activity was confirmed by stimulating the proliferation of TF1 cell line. This study demonstrated that Y. lipolytica is a promising host for the efficient production of active toxic proteins like hGM-CSF.






Similar content being viewed by others
References
Armitage JO (1985) Emerging applications of recombinant human granulocyte–macrophage colony-stimulating factor. EMBO J 4:2575–2581
Armitage JO (1998) Emerging applications of recombinant human granulocyte–macrophage colony-stimulating factor. Blood 92(12):4491–4508
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol 19:219–237
Bergès H, Joseph-Liauzun E, Fayet O (1996) Combined effects of the signal sequence and the major chaperone proteins on the export of human cytokines in Escherichia coli. Appl Environ Microbiol 62(1):55–60
Cebon J, Nicola N, Ward M, Gardner I, Dempsey P, Layton J, Dührsen U, Burgess AW, Nice E, Morstyn G (1990) Granulocyte–macrophage colony stimulating factor from human lymphocytes. The effect of glycosylation on receptor binding and biological activity. J Biol Chem 265(8):4483–91
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24(1):45–66
Chiou CJ, Wu MC (1990) Expression of human granulocyte–macrophage colony-stimulating factor gene in insect cells by a baculovirus vector. FEBS Lett 259(2):249–53
Das KM, Banerjee S, Shekhar N, Damodaran K, Nair R, Somani S, Raiker VP, Jain S, Padmanabhan S (2011) Cloning, soluble expression and purification of high yield recombinant hGM-CSF in Escherichia coli. Int J Mol Sci 12(3):2064–76
Dedhar S, Gaboury L, Galloway P, Eaves C (1988) Human granulocyte–macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci 85(23):9253–7
DeLamarter JF, Mermod JJ, Liang CM, Eliason JF, Thatcher DR (1985) Recombinant murine GM-CSF from E.coli has biological activity and is neutralized by a specific antiserum. EMBO J 4:2575–2581
Dorr RT (1993) Clinical properties of yeast-derived versus Escherichia coli-derived granulocyte–macrophage colony-stimulating factor. Clin Ther 15(1):19–29
Fickers P, Le Dall MT, Gaillardin C, Tonart P, Nicaud JM (2003) New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipoytica. J Microbial Methods 55:727–737
Forno G, Bollati Fogolin M, Oggero M, Kratje R, Etcheverrigaray M, Conradt HS, Nimtz M (2004) N- and O-linked carbohydrates and glycosylation site occupancy in recombinant human granulocyte–macrophage colony-stimulating factor secreted by a Chinese hamster ovary cell line. Eur J Biochem 271(5):907–19
Gasmi N, Ayed A, Nicaud JM, Kallel H (2011a) Design of an efficient medium for heterologous protein production in Yarrowia lipolytica: case of human interferon alpha 2b. Microb Cell Fact 10:38
Gasmi N, Fudalej F, Kallel H, Nicaud JM (2011b) A molecular approach to optimize hIFN α2b expression and secretion in Yarrowia lipolytica. Appl Microbiol Biotechnol 89(1):109–19
James EA, Wang C, Wang Z, Reeves R, Shin JH, Magnuson NS, Lee JM (2000) Production and characterization of biologically active human GM-CSF secreted by genetically modified plant cells. Protein Expr Purif 19(1):131–8
Khasa YP, Khushoo A, Tapryal S, Mukherjee KJ (2011) Optimization of human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression using asparaginase and xylanase gene's signal sequences in Escherichia coli. Appl Biochem Biotechnol 165(2):523–37
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lu D, Yang C, Liu Z (2012) How hydrophobicity and the glycosylation site of glycans affect protein folding and stability: a molecular dynamics simulation. J Phys Chem B116(1):390–400
Madzak C, Gaillardin C, Beckerich JM (2004) Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica. J Biotechnol 109:63–81
McGrew JT, Leiske D, Dell B, Klinke R, Krasts D, Wee SF, Abbott N, Armitage R, Harrington K (1997) Expression of trimeric CD40 ligand in Pichia pastoris: use of a rapid method to detect high-level expressing transformants. Gene 18:193–200
Metcalf D (1985) The granulocyte–macrophage colony-stimulating factors. Science 229(4708):16–22
Miyajima A, Otsu K, Schreurs J, Bond MW, Abrams JS, Arai K (1986) Expression of murine and human granulocyte–macrophage colony-stimulating factors in S. cerevisiae: mutagenesis of the potential glycosylation sites. EMBO J 5(6):1193–7
Modrowski D, Lomri A, Marie PJ (1998) Glycosaminoglycans bind granulocyte–macrophage colony-stimulating factor and modulate its mitogenic activity and signaling in human osteoblastic cells. J Cell Physiol 177(1):187–95
Moonen P, Mermod JJ, Ernst JF, Hirschi M, DeLamarter JF (1987) Increased biological activity of deglycosylated recombinant human granulocyte/macrophage colony-stimulating factor produced by yeast or animal cells. Proc Natl Acad Sci USA 84:4428–4431
Petersen TN, Brunak S, Heijne GV, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods 8:785–786
Pignède G, Wang HJ, Fudalej F, Seman M, Gaillardin C, Nicaud JM (2000) Autocloning and amplification of LIP2 in Yarrowia lipolytica. Appl Environ Microbiol 66:3283–9
Sahdev S, Khattar SK, Saini KS (2008) Production of active eukaryotic proteins through bacterial expression system: a review of the existing biotechnology strategies. Mol Cell Biochem 307:249–264
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, New York
Schwanke RC, Renard G, Chies JM, Campos MM, Batista EL Jr, Santos DS, Basso LA (2009) Molecular cloning, expression in Escherichia coli and production of bioactive homogeneous recombinant human granulocyte and macrophage colony stimulating factor. Int J Biol Macromol 45(2):97–102
Srinivasa BK, Antony A, Muthukumaran T, Meenakshisundaram S (2008) Construction of intein-mediated hGMCSF expression vector and its purification in Pichia pastoris. Protein Expr Purif 57(2):201–5
Srinivasa BK, Muthukumaran T, Antony A, Samuel SDPS, Balamurali M, Murugan V, Meenakshisundaram S (2009) Single step intein-mediated purification of hGM-CSF expressed in salt-inducible E. coli. Biotechnol Lett 31:659–664
Yin J, Li G, Ren X, Herrler GA (2007) Comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol 127:335–347
Acknowledgments
The authors would like to acknowledge EGIDE for their financial support in the frame of the CMCU project France–Tunisia (grant no. 07 G0913). LC-MS/MS analyses were performed using PAPPSO (Plateforme d’Analyses Protéomiques Paris Sud Ouest, http://pappso.inra.fr) facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gasmi, N., Lassoued, R., Ayed, A. et al. Production and characterization of human granulocyte–macrophage colony-stimulating factor (hGM-CSF) expressed in the oleaginous yeast Yarrowia lipolytica . Appl Microbiol Biotechnol 96, 89–101 (2012). https://doi.org/10.1007/s00253-012-4141-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4141-x