Abstract
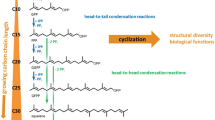
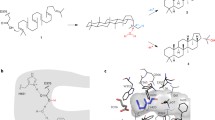
Squalene-hopene cyclases (SHCs) are prokaryotic enzymes that catalyse the cyclisation of the linear precursor squalene to pentacyclic hopene. Recently, we discovered that a SHC cloned from Zymomonas mobilis (ZMO-1548 gene product) has the unique property to cyclise the monoterpenoid citronellal to isopulegol. In this study, we performed saturation mutagenesis of three amino acids of the catalytic centre of ZMO-1548 (F428, F486 and W555), which had been previously identified to interact with enzyme-bound substrate. Replacement of F428 by tyrosine increased hopene formation from squalene, but isopulegol-forming activity was strongly reduced or abolished in all muteins of position 428. W555 was essential for hopene formation; however, three muteins (W555Y, W428F or W555T) revealed enhanced cyclisation efficiency with citronellal. The residue at position 486 turned out to be the most important for isopulegol-forming activity. While the presence of phenylalanine or tyrosine favoured cyclisation activity with squalene, several small and/or hydrophobic residues such as cysteine, alanine or isoleucine and others reduced activity with squalene but greatly enhanced isopulegol formation from citronellal. Replacement of the conserved aromatic residue corresponding to F486 to cysteine in other SHCs cloned from Z. mobilis (ZMO-0872), Alicyclobacillus acidocaldarius (SHC Aac ), Acetobacter pasteurianus (SHC Apa ), Streptomyces coelicolor (SHC Sco ) and Bradyrhizobium japonicum (SHC Bja ) resulted in more or less strong isopulegol-forming activities from citronellal. In conclusion, many SHCs can be converted to citronellal cyclases by mutagenesis of the active centre thus broadening the applicability of this interesting class of biocatalyst.





Similar content being viewed by others
References
Abe I (2007) Enzymatic synthesis of cyclic triterpenes. Nat Prod Rep 24:1311
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A 95:4126–4133
Davis EM, Croteau R (2000) Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Topic Curr Chem 209:53–95
Greenhagen B, Chappell J (2001) Molecular scaffolds for chemical wizardry: learning nature’s rules for terpene cyclases. Proc Natl Acad Sci U S A 98:13479–13481
Hoshino T, Kumai Y, Kudo I, Nakano S-I, Ohashi S (2004a) Enzymatic cyclization reactions of geraniol, farnesol and geranylgeraniol, and those of truncated squalene analogs having C20 and C25 by recombinant squalene cyclase. Org Biomol Chem 2:2650–2657
Hoshino T, Kumai Y, Sato T (2009) Reviewing the polyolefin cyclization reaction of the C35 polyprene catalyzed by squalene-hopene cyclase. Chemistry 15:2091–2100
Hoshino T, Nakano S-I, Kondo T, Sato T, Miyoshi A (2004b) Squalene-hopene cyclase: final deprotonation reaction, conformational analysis for the cyclization of (3R, S)-2,3-oxidosqualene and further evidence for the requirement of an isopropylidene moiety both for initiation of the polycyclization cascade and for the formation of the 5-membered E-ring. Org Biomol Chem 2:1456–1470
Hoshino, T. & Sato, T. (2002). Squalene-hopene cyclase: catalytic mechanism and substrate recognition. Chem Commun (Camb) 291–301.
Kannenberg EL, Poralla K (1999) Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 86:168–176
Lange BM (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci U S A 97:13172–13177
Miziorko HM (2011) Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys 505:131–143
Ourisson G, Rohmer M, Poralla K (1987) Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Ann Rev Microbiol 41:301–333
Sato T, Hoshino T (1999) Kinetic studies on the function of all the conserved tryptophans involved inside and outside the QW motifs of squalene-hopene cyclase: stabilizing effect of the protein structure against thermal denaturation. Biosci Biotechnol Biochem 63:1171–1180
Sato T, Hoshino T (2001) Catalytic function of the residues of phenylalanine and tyrosine conserved in squalene-hopene cyclases. Biosci Biotechnol Biochem 65:2233–2242
Sato T, Kouda M, Hoshino T (2004) Site-directed mutagenesis experiments on the putative deprotonation site of squalene-hopene cyclase from Alicyclobacillus acidocaldarius. Biosci Biotechnol Biochem 68:728–738
Seitz, M., Klebensberger, J., Siebenhaller, S., Breuer, M., Siedenburg, G., Jendrossek, D. & Hauer, B. 2012. Substrate specificity of a novel squalene-hopene cyclase from Zymomonas mobilis. J Mol Catal B: Enzym. doi:10.1016/j.molcatb.2012.02.007
Siedenburg G, Jendrossek D (2011) Squalene-hopene cyclases. Appl Environ Microbiol 77:3905–3915
Siedenburg G, Jendrossek D, Breuer M, Juhls B, Pleiss J, Seitz M, Klebensberger J, Hauer B (2012) Activation-independent cyclization of monoterpenoids. Appl Environ Microbiol 78:1055–1062
Smentek L, Hess BA (2010) Compelling computational evidence for the concerted cyclization of the ABC Rings of hopene from protonated squalene. J Am Chem Soc 132:17111–17117
Sprenger GA, Schörken U, Wiegert T, Grolle S, de Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H (1997) Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-D-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci U S A 94:12857–12862
Tholl D (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 9:297–304
Wendt KU, Lenhart A, Schulz GE (1999) The structure of the membrane protein squalene-hopene cyclase at 2.0 Å resolution 1. J Mol Biol 286:175–187
Wendt KU, Poralla K, Schulz GE (1997) Structure and function of a squalene cyclase. Science 277:1811–1815
Wendt KU (2005) Enzymatische Triterpen-Cyclisierung: neue Mosaikbausteine vervollständigen das Bild. Angew Chem 117:4032–4037
Yonemura Y, Ohyama T, Hoshino T (2012) Chemo-enzymatic syntheses of drimane-type sesquiterpenes and the fundamental core of hongoquercin meroterpenoid by recombinant squalene-hopene cyclase. Org Biomol Chem 10:440–446
Acknowledgments
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF, reference number: 315406).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siedenburg, G., Breuer, M. & Jendrossek, D. Prokaryotic squalene-hopene cyclases can be converted to citronellal cyclases by single amino acid exchange. Appl Microbiol Biotechnol 97, 1571–1580 (2013). https://doi.org/10.1007/s00253-012-4008-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4008-1