Abstract
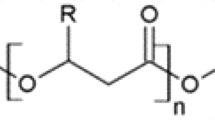
Thermally synthesized poly(aspartate) (tPAA) shows potential for use in a wide variety of products and applications as a biodegradable replacement for non-biodegradable polycarboxylates, such as poly(acrylate). The tPAA molecule has unnatural structures, and the relationship between its biodegradability and structures has been investigated. Two tPAA-degrading bacteria, Sphingomonas sp. KT-1 and Pedobacter sp. KP-2, were isolated from river water; from them, two PAA-hydrolyzing enzymes, PAA hydrolases-1 and -2, were purified and biologically and genetically characterized. Interestingly, not only are PAA hydrolases-1 from those two strains novel in terms of structural genes and substrate specificities (they specifically cleave the amide bond between β-aspartate units in tPAA), they also probably play a central role in tPAA biodegradation by both strains. In green polymer chemistry, one active area of research is the use of purified enzymes for the enzyme-catalyzed synthesis of polypeptides by taking advantage of their substrate specificities. Recently, β-peptides have attracted academic and industrial interest as functional materials as they possess both functions of α-peptides and excellent metabolic stability. As one of the attractive applications of PAA hydrolases, we report here the enzyme-catalyzed synthesis of poly(α-ethyl β-aspartate), which is composed of only β-linkages and belongs to β-peptides, using the unique substrate specificity of the enzyme from Pedobacter sp. KP-2.





Similar content being viewed by others
References
Aso K, Uemura T, Shiokawa Y (1988) Protease-catalyzed synthesis of oligo-l-glutamic acid from l-glutamic acid diethyl ester. Agric Biol Chem 52:2443–2449
Freeman MB, Paik YH, Swift G, Wilczynski R, Wolk SK, Yocom KM (1996) Biodegradability of polycarboxylates: structure–activity studies. In: Ottenbrite RM, Huang SJ, Park K (eds) Hydrogels and biodegradable polymers for bioapplications, ACS Symposium Series. American Chemical Society, Washington, pp 118–136
Guichard G (2004) β-Peptides, γ-peptides and isosteric backbones: new scaffolds with controlled shapes for mimicking protein secondary structure elements. In: Nielsen PE (ed) Pseudo-peptides in drug discovery. Wiley-VCH, Weinheim, pp 33–120
Heck T, Kohler HPE, Limbach M, Flcgel O, Seebach D, Geueke B (2007) Enzyme-catalyzed formation of β-peptides: β-peptidyl aminopeptidases BapA and DmpA acting as β-peptide-synthesizing enzymes. Chem Biodiv 4:2016–2030
Hiraishi T, Taguchi S (2009) Enzyme-catalyzed synthesis and degradation of biopolymers. Mini-Rev Org Chem 6:44–54
Hiraishi T, Kajiyama M, Tabata K, Yamato I, Doi Y (2003a) Genetic analysis and characterization of poly(aspartic acid) hydrolase-1 from Sphingomonas sp. KT-1. Biomacromolecules 4:80–86
Hiraishi T, Kajiyama M, Tabata K, Abe H, Yamato I, Doi Y (2003b) Biochemical and molecular characterization of poly(aspartic acid) hydrolase-2 from Sphingomonas sp. KT-1. Biomacromolecules 4:1285–1292
Hiraishi T, Kajiyama M, Yamato I, Doi Y (2004) Enzymatic hydrolysis of α- and β-oligo(l-aspartic acid)s by poly(aspartic acid) hydrolases-1 and 2 from Sphingomonas sp. KT-1. Macromol Biosci 4:330–339
Hiraishi T, Masuda E, Kanayama N, Nagata M, Doi Y, Abe H, Maeda M (2009) Cloning of poly(aspartic acid) (PAA) hydrolase-1 gene from Pedobacter sp. KP-2 and hydrolysis of thermally synthesized PAA by its gene product. Macromol Biosci 9:10–19
Hiraishi T, Masuda E, Miyamoto D, Kanayama N, Abe H, Maeda M (2011) Enzymatic synthesis of poly(α-ethyl β-aspartate) by poly(ethylene glycol) modified poly(aspartate) hydrolase-1. Macromol Biosci 11:187–191
Joentgen W, Müller N, Mitschker A, Schmidt H (2003) Polyaspartic acids: polyamides and complex proteinaceous materials I. In: Fahnestock SR, Steinbüchel A (eds) Biopolymers 7. Wiley-VCH, Weinheim, pp 175–199
Kaplan DL, Dordick J, Gross RA, Swift G (1998) Enzymes in polymer science: an introduction. In: Gross RA, Kaplan DL, Swift G (eds) Enzymes in polymer synthesis, ACS Symposium Series 684. American Chemical Society, Washington, p 2
Kawai F (1999) Sphingomonads involved in the biodegradation of xenobiotic polymers. J Ind Microbiol Biotechnol 23:400–407
Kawai F (2010) The biochemistry and molecular biology of xenobiotic polymer degradation by microorganisms. Biosci Biotechnol Biochem 74:1743–1759
Kricheldorf HR (2006) Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew Chem Int Ed 45:5752–5784
Li G, Vaidya A, Viswanathan K, Cui J, Xie W, Gao W, Gross RA (2006) Rapid regioselective oligomerization of l-glutamic acid diethyl ester catalyzed by papain. Macromolecules 39:7915–7921
Li G, Raman VK, Xie W, Gross RA (2008) Protease-catalyzed co-oligomerizations of l-leucine ethyl ester with l-glutamic acid diethyl ester: sequence and chain length distributions. Macromolecules 41:7003–7012
Low KC, Wheeler AP, Koskan LP (1996) Commercial poly(aspartic acid) and its uses. In: Glass J (ed) Hydrophilic polymers. Advances in Chemistry series. American Chemical Society, Washington, pp 99–111
Matsubara K, Nakato T, Tomida M (1998) End group and irregular structure analysis in thermally prepared sodium polyaspartate by 1H and 13C NMR spectroscopy. Macromolecules 31:1466–1472
Matsumura S, Tsushima Y, Otozawa N, Murakami S, Toshima K, Swift G (1999) Enzyme-catalyzed polymerization of l-aspartate. Macromol Rapid Commun 20:7–11
Nakato T, Yoshitake M, Matsubara K, Tomida M, Kakuchi T (1998) Relationships between structure and properties of poly(aspartic acid)s. Macromolecules 31:2107–2113
Obst M, Oppermann-Sanio FB, Luftmann H, Steinbüchel A (2002) Isolation of cyanophycin-degrading bacteria, cloning and characterization of an extracellular cyanophycinase gene (cphE) from Pseudomonas anguilliseptica strain BI. J Biol Chem 277:25096–25105
Obst M, Sallam A, Luftmann H, Steinbüchel A (2004) Isolation and characterization of Gram-positive cyanophycin-degrading bacteria—kinetic studies on cyanophycin depolymerase activity in aerobic bacteria. Biomacromolecules 5:153–161
Osada K, Christie RJ, Kataoka K (2009) Polymeric micelles from poly(ethylene glycol)–poly(amino acid) block copolymer for drug and gene delivery. J R Soc Interface 6:S325–S339
Paik YH, Simon ES, Swift G (1996) A review of synthetic approaches to biodegradable polymeric carboxylic acids for detergent applications. In: Glass J (ed) Hydrophilic polymers. Advances in Chemistry series. American Chemical Society, Washington, pp 79–98
Pivcova H, Saudek V, Drobnik J, Vlasak J (1981) NMR study of poly(aspartic acid). I. α- and β-Peptide bonds in poly(aspartic acid) prepared by thermal polycondensation. Biopolymers 20:1605–1614
Pivcova H, Saudek V, Drobnik J (1982) 13C NMR study of the structure of poly(aspartic acid). Polymer 23:1237–1241
Richter R, Hejazi M, Kraft R, Ziegler K, Lockau W (1999) Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Eur J Biochem 263:163–169
Ross RJ, Mazo GY, Mazo J (2001) New methods in the synthesis of thermal poly(aspartates). In: Gross R, Scholz C (eds) Biopolymers from polysaccharides and agroproteins, ACS Symposium Series. American Chemical Society, Washington, pp 172–181
Sallam A, Kast A, Przybilla S, Meiswinkel T, Steinbüchel A (2009) Biotechnological process for production of β-dipeptides from cyanophycin on a technical scale and its optimization. Appl Environ Microbiol 75:29–38
Schwab LW, Baum I, Fels G, Loosa K (2010) Mechanistic insight in the enzymatic ring-opening polymerization of β-propiolactam. In: Cheng HN, Gross R (eds) Green polymer chemistry: biocatalysis and biomaterials, ACS Symposium Series. American Chemical Society, Washington, pp 265–278
Seebach D, Gardiner J (2008) β-Peptidic peptidomimetics. Acc Chem Res 41:1366–1375
Seebach D, Beck AK, Bierbaum DJ (2004) The world of β- and γ-peptides comprised of homologated proteinogenic amino acids and other components. Chem Biodiversity 1:1111–1239
Soeda Y, Toshima K, Matsumura S (2003) Sustainable enzymatic preparation of polyaspartate using a bacterial protease. Biomacromolecules 4:196–203
Tabata K, Kasuya K, Abe H, Masuda K, Doi Y (1999) Poly(aspartic acid) degradation by a Sphingomonas sp. isolated from freshwater. App Environ Microbiol 65:4268–4270
Tabata K, Abe H, Doi Y (2000) Microbial degradation of poly(aspartic acid) by two isolated strains of Pedobacter sp. and Sphingomonas sp. Biomacromolecules 1:157–161
Tabata K, Kajiyama M, Hiraishi T, Abe H, Yamato I, Doi Y (2001) Purification and characterization of poly(aspartic acid) hydrolase from Sphingomonas sp. KT-1. Biomacromolecules 2:1155–1160
Tang Y, Wheeler AP (2001) Environmental factors that influence biodegradation of thermal poly(aspartate). In: Gross R, Scholz C (eds) Biopolymers from polysaccharides and agroproteins, ACS Symposium Series. American Chemical Society, Washington, pp 157–171
Thombre SM, Sarwade BD (2005) Synthesis and biodegradability of polyaspartic acid: a critical review. J Macromol Sci, Part A: Pure Appl Chem 42:1299–1315
Uemura T, Fujimori M, Lee HH, Ikeda S, Aso K (1990) Polyethylene glycol-modified papain catalyzed oligopeptide synthesis from the esters of l-aspartic and l-glutamic acids in benzene. Agric Biol Chem 54:2277–2281
Uyama H, Fukuoka T, Komatsu I, Watanabe T, Kobayashi S (2002) Protease-catalyzed regioselective polymerization and copolymerization of glutamic acid diethyl ester. Biomacromolecules 3:318–323
Wolk SK, Swift G, Paik YH, Yocom KM, Smith RL, Simon ES (1994) One- and two-dimensional nuclear magnetic resonance characterization of poly(aspartic acid) prepared by thermal polymerization of l-aspartic acid. Macromolecules 27:7613–7620
Acknowledgments
We would like to sincerely thank Professor A. Steinbüchel for granting us the opportunity to prepare this mini-review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hiraishi, T., Maeda, M. Poly(aspartate) hydrolases: biochemical properties and applications. Appl Microbiol Biotechnol 91, 895–903 (2011). https://doi.org/10.1007/s00253-011-3429-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3429-6