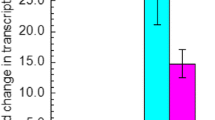
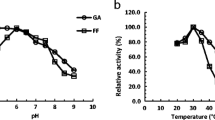
Abstract
Furfural and 5-hydroxymethylfurfural (HMF) are representative inhibitors generated from biomass pretreatment using dilute acid hydrolysis that interfere with yeast growth and subsequent fermentation. Few yeast strains tolerant to inhibitors are available. In this study, we report a tolerant strain, Saccharomyces cerevisiae NRRL Y-50049, which has enhanced biotransformation ability to convert furfural to furan methanol (FM), HMF to furan di-methanol (FDM), and produce a normal yield of ethanol. Our recent identification of HMF and development of protocol to synthesize the HMF metabolic conversion product FDM allowed studies on fermentation metabolic kinetics in the presence of HMF and furfural. Individual gene-encoding enzymes possessing aldehyde reduction activities demonstrated cofactor preference for NADH or NADPH. However, protein extract from whole yeast cells showed equally strong aldehyde reduction activities coupled with either cofactor. Deletion of a single candidate gene did not affect yeast growth in the presence of the inhibitors. Our results suggest that detoxification of furfural and HMF by the ethanologenic yeast S. cerevisiae strain Y-50049 likely involves multiple gene mediated NAD(P)H-dependent aldehyde reduction. Conversion pathways of furfural and HMF relevant to glycolysis and ethanol production were refined based on our findings in this study.





Similar content being viewed by others
References
Aguilera J, Prieto JA (2001) The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr Genet 39:273–83
Antal MJ, Leesomboon T, Mok WS, Richards GN (1991) Mechanism of formation of 2-furaldehyde from D-xylose. Carbohyd Res 217:71–85
Aranda A, del Olmo M (2003) Response to acetaldehyde stress in the yeast Saccharomyces cerevisiae involves a strain-dependent regulation of several ALD genes and is mediated by the general stress response pathway. Yeast 20:747–59
Banerjee N, Bhatnagar R, Viswanathan L (1981) Development of resistance in Saccharomyces cerevisiae against inhibitory effects of Browning reaction products. Enzy Microb Technol 3:24–28
Bothast R, Saha BC (1997) Ethanol production from agricultural biomass substrate. Adv Appl Microbiol 44:261–286
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chung IS, Lee YY (1985) Ethanol fermentation of crude acid hydrolyzate of cellulose using high-level yeast inocula. Biotechnol Bioeng 27:308–315
Cottier L, Desotes G, Soro Y (2003) Heteromacrocycles from ring-closing metathesis of unsaturated furanic ethers. Synthetic Comm 33:4285–4295
Dunlop AP (1948) Furfural formation and behavior. Ind Eng Chem 40:204–209
Gutierrez T, Buszko ML, Ingram LO, Preston JF (2002) Reduction of furfural and furfuryl alcohol by ethanologenic strains of bacteria and its effect on ethanol production from xylose. Appl Biochem Biotechnol 98–100:327–340
Gutierrez T, Ingram LO, Preston JF (2006) Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LYO1—An enzyme important in the detoxification of furfural during ethanol production. J Biotechnol 121:154–164
Heidlas J, Tressl R (1990) Purification and characterization of a (R)-2,3-bytanediol dehydrogenase from Saccharomyces cerevisiae. Arch Microbiol 154:267–273
Iovel I, Goldberg Y, Shymanska M (1989) Hydroxymethylation of furan and its derivatives in the presence of cation-exchange resins. J Mol Catalysis 57:91–103
Kalapos MP (1999) Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110:145–75
Khan Q, Hadi S (1994) Inactivation and repair of bacteriophage lambda by furfural. Biochem Mol Biol Int 32:379–385
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–16
Kuyper M, Toirkens MJ, Diderich JA, Winkler AA, van Dijken JP, Pronk JT (2005) Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res 5:925–934
Larroy C, Fernadez MR, Gonzalez E, Pares X, Biosca JA (2002a) Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH-dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J 361:163–172
Larroy C, Pares X, Biosca JA (2002b) Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur J Biochem 269:5738–5745
Larroy C, Rosario FM, Gonzalez E, Pares X, Biosca JA (2003) Properties and functional significance of Saccharomyces cerevisiae ADHVI. Chem Biol Interact 143–144:229–238
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N (1999) The Generation of inhibitors during dilute acid hydrolysis of softwood. Enzy Micro Technol 24:151–159
Lewkowski J (2001) Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. Arkivoc 1:17–54
Li u ZL (2006) Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol 73:27–36
Liu ZL, Slininger PJ (2005) Development of genetically engineered stress tolerant ethanologenic yeasts using integrated functional genomics for effective biomass conversion to ethanol. In: Outlaw J, Collins K, Duffield J (eds) Agriculture as a producer and consumer of energy. CAB International, Wallingford, pp 283–294
Liu ZL, Slininger PJ (2006) Transcriptome dynamics of ethanologenic yeast in response to 5-hydroxymethylfurfural stress related to biomass conversion to ethanol. In: MarzalA., ReviriegoM. I., NavarroC., de LopeF., MøllerA. P. (eds) Recent research developments in multidisciplinary applied microbiology: understanding and exploiting microbes and their interactions-biological, physical, chemical and engineering aspects. Wiley, New York, pp 679–684
Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurtzman CP, Gorsich SW (2004) Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol 31:345–352
Liu ZL, Slininger PJ, Gorsich SW (2005) Enhanced biotransformation of furfural and 5-hydroxy methylfurfural by newly developed ethanologenic yeast strains. Appl Biochem Biotechnol 121–124:451–460
Marteniz A, Rodriguez ME, York SW, Preston JF, Ingram LO (2000) Effect of Ca(OH)2 treatments (“Overeliming”) on the composition and toxicity of bagasse hemicellulose hydrolyzates. Biotechnol Bioeng 69:526–536
Martin C, Jonsson L (2003) Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocellulose-derived fermentation inhibitors. Enzy Micro Technol 32:386–395
Martin C, Marcelo M, Almazan O, Jonsson LJ (2007) Adaptation of a recombinant xylose-utilizing Saccharomyces cerevisiae strain to a sugarcane bagasse hydrolysate with high content of fermentation inhibitors. Biores Technol 98:1767–1773
Modig T, Liden G, Taherzadeh M (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776
Morimoto S, Murakami M (1967) Studies on fermentation products from aldehyde by microorganisms: the fermentative production of furfural alcohol from furfural by yeasts (part I). J Ferm Technol 45:442–446
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of dilute-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93:1–10
Nemeikaite-Ceniene A, Sarlauskas J, Miseviciene L, Anusevicius Z, Maroziene A, Cenas N (2004) Enzymatic redox reactions of the explosive 4,6-dinitrobenzofuroxan (DNBF): implications for its toxic action. Acta Biochim Pol 51:1081–1086
Nemirovskii V, Kostenko V (1991) Transformation of yeast growth inhibitors which occurs during biochemical processing of wood hydrolysates. Gidroliz Lesokhimm Prom-st 1:16–17
Nemirovskii V, Gusarova L, Rakhmilevich Y, Sizov A, Kostenko V (1989) Pathways of furfurol and oxymethyl furfurol conversion in the process of fodder yeast cultivation. Biotekhnologiya 5:285–289
Nilsson A, Gorwa-Grauslund MF, Hahn-Hagerdal B, Liden G (2005) Cofactor dependence in furan reduction by Saccharomyces cerevisiae in fermentation of acid-hydrolyzed lignocellulose. App Environ Microbiol 71:7866–7871
Outlaw J, Collins KJ, Duffield JA (2005) Agriculture as a producer and consumer of energy. CABI, Oxfordshire
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Petersson A, Almeida JRM, Modig T, Karhumaa K, Hahn-Hägerdal B, Gorwa-Grauslund MF, Liden G (2006) A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 23:455–464
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sambrook J, Russell DW (2001) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanchez B, Bautista J (1988) Effects of furfural and 5-Hydroxymethylfurfrual on the fermentation of Saccharomyces cerevisiae and biomass production from Candida uilliermondii. Enzy Micro Technol 10:315–318
Song MJ, Liu ZL (2007) A linear discrete dynamic system model for temporal gene interaction and regulatory network influence in response to bioethanol conversion inhibitor HMF for ethanologenic yeast. Lect Notes Bioinfomatics 4532:77–95
Taherzadeh M, Gustafsson L, Niklasson C (2000) Physiological effects of 5-Hydroxymethylfurfural on Saccharomyces cerevisiae. App Microbiol Biotechnol 53:701–708
Tessier WD, Meaden PG, Dickinson FM, Midgley M (1998) Identification and disruption of the gene encoding the K(+)-activated acetaldehyde dehydrogenase of Saccharomyces cerevisiae. FEMS Microbiol Lett 164:29–34
Träff KL, Otero Cordero RR, van Zyl WH, Hahn-Hägerdal B (2001) Deletion of the GRE3 aldose reductase gene and its influence on xylose metabolism in recombinant strains of Saccharomyces cerevisiae expression the xylA and XKSI genes. Appl Environ Microbiol 67:5668–5674
Träff KL, Jönsson LJ, Hahn-Hägerdal, B (2002) Putative xylose and arabinose reductases in Saccharomyces cerevisiae. Yeast 19:1233–1241
Villa GP, Bartroli R, Lopez R, Guerra M, Enrique M, Penas M, Rodriquez E, Redondo D, Iglesias I, Diaz I (1992) Microbial transformation of furfural to furfuryl alcohol by Saccharomyces cerevisiae. Acta Biotechnol 12:509–512
Wahlbom CF, Hahn-Hägerdal B (2002) Furfural, 5-hydroxymethylfurfrual, and acetone act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng 78:172–178
Wheals AE, Basso LC, Alves DM, Amorim HV (1999) Fuel ethanol after 25 years. Trends Biotechnol 17:482–487
Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Davis RW et al (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Acknowledgments
We are grateful for technical assistance contributed by Amy Cash, Suzanne Milborne, and Maureen Shea-Andersh. This study was supported by US Department of Agriculture ARS National Programs 307/306 and, in part, supported by USDA HQ Mentor of Postdoctoral Research Associate Program and the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2006-35504-17359 to ZLL.
Author information
Authors and Affiliations
Corresponding author
Additional information
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Lewis Liu, Z., Moon, J., Andersh, B.J. et al. Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxymethylfurfural by Saccharomyces cerevisiae . Appl Microbiol Biotechnol 81, 743–753 (2008). https://doi.org/10.1007/s00253-008-1702-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1702-0