Abstract
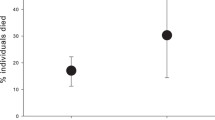
Research on immune function in evolutionary ecology has frequently focused on avian ectoparasites (e.g., mites and lice). However, host immunogenetics involved with bird resistance to ectoparasites has not been determined. The critical role of the major histocompatibility complex (MHC) in adaptive immunity and high genetic variation found within the MHC make this gene complex useful for exploring the immunogenetic basis for bird resistance to ectoparasites. The objective of this study was to determine if the avian MHC influenced resistance to a blood-feeding ectoparasite. Four congenic lines of chickens, differing only at the MHC, were comparatively infested with a cosmopolitan ectoparasite of birds—northern fowl mite (NFM)—which is also a serious pest species of poultry. Mite infestations were monitored over time and mite densities (weekly and maximum) were compared among lines. Chickens with the MHC haplotype B21 were relatively resistant to NFM, compared with birds in the B15 congenic line (P < 0.02). To test for similar effects in an outbred genetic background, a separate experiment was performed with 107 commercial chickens (white leghorn, W-36 strain) infested with NFM. Hens were genotyped using a MHC microsatellite marker (LEI0258) and associations between MHC haplotype and NFM density were tested. The highest peak NFM populations occurred more often on hens with the B15 haplotype versus the B21 haplotype (P = 0.012), which supported the results of the congenic study. These data indicate the avian MHC influences ectoparasite resistance, which is relevant to disease ecology and avian–ectoparasite interaction.




Similar content being viewed by others
References
Abplanalp H (1992) Inbred lines as genetic resources of chickens. Poult Sci Rev 4:29–39
Acosta-Rodriguez R, Alonso-Morales R, Balladares S, Flores-Aguilar H, Garcia-Vazquez Z, Gorodezky C (2005) Analysis of BoLA class II microsatellites in cattle infested with Boophilus microplus ticks: class II is probably associated with susceptibility. Vet Parasitol 127:313–321 doi:10.1016/j.vetpar.2004.10.007
Aeed PA, Briles WE, Zsigray RM, Collins WM (1993) Influence of different B-complex recombinants on the outcome of Rous sarcomas in chickens. Anim Genet 24:177–181
Apanius V, Penn D, Slev PR, Ruff LR, Potts WK (1997) The nature of selection on the major histocompatibility complex. Crit Rev Immunol 17:179–224
Arthur FH, Axtell RC (1982) Comparisons of permethrin formulations and application methods for northern fowl mite control on caged laying hens. Poult Sci 61:879–884
Axtell RC, Arends JJ (1990) Ecology and management of arthropod pests of poultry. Annu Rev Entomol 35:101–126 doi:10.1146/annurev.en.35.010190.000533
Axtner J, Sommer S (2007) Gene duplication, allelic diversity, selection processes and adaptive value of MHC class II DRB genes of the bank vole, Clethrionomys glareolus. Immunogenetics 59:417–426 doi:10.1007/s00251-007-0205-y
Billingsley PF, Baird J, Mitchell JA, Drakeley C (2006) Immune interactions between mosquitoes and their hosts. Parasite Immunol 28:143–153
Blanco G, De la Puente J, Corroto M, Baz T, Colas J (2001) Condition-dependent immune defense in the Magpie: how important is ectoparasitism? Biol J Linn Soc 72:279–286
Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G (2004) Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution Int J Org Evolution 58:2823–2830
Bonneaud C, Perez-Tris J, Federici P, Chastel O, Sorci G (2006) Major histocompatibility alleles associated with local resistance to malaria in a passerine. Evolution Int J Org Evolution 60:383–389
Boonyanuwat K, Thummabutra S, Sookmanee N, Vatchavalkhu V, Siripholvat V (2006) Influences of major histocompatibility complex class I haplotypes on avian influenza virus disease traits in Thai indigenous chickens. Anim Sci J 77:285–289 doi:10.1111/j.1740-0929.2006.00350.x
Briles WE, Stone HA, Cole RK (1977) Mareks disease effects of B-histocompatibility alloalleles in resistant and susceptible chicken lines. Science 195:193–195 doi:10.1126/science.831269
Brodsky FM (2001) Antigen presentation & the major histocompatibility complex. In: Parslow TG, Stites DP, Terr AI, Imboden JB (eds) Medical immunology. McGraw-Hill, New York, pp 82–94
Brossard M, Wikel SK (2004) Tick immunobiology. Parasitology 129:S161–S176 doi:10.1017/S0031182004004834
Burg JG, Collison CH, Mastro AM (1988) Comparative analysis of precipitating antibodies in White Rock and Fayoumi hens injected with bovine serum albumin or crude mite extract with resulting effects on northern fowl mite, Ornithonyssus sylviarum (Acari: Macronyssidae) population densities. Poult Sci 67:1015–1019
Cheeseman JH, Kaiser MG, Lamont SJ (2004) Genetic line effect on peripheral blood leukocyte cell surface marker expression in chickens. Poult Sci 83:911–916
Davison TF, Morris TR, Payne LN (eds) (1996) Poultry immunology. Poultry science symposium series. Carfax, London
Deloach JR, DeVaney JA (1981) Nothern fowl mite, Ornithonyssus sylviarum (Acari, Macronyssidae), ingests large quantities of blood from white leghorn hens. J Med Entomol 18:374–377
DeVaney JA, Martin BW (1984) Factors affecting northern fowl mite populations on chickens: effect of age of pullet at time of infestation and effect of caponizing roosters. Poult Sci 63:1327–1332
DeVaney JA, Ziprin RL (1980) Acquired immune response of white leghorn hens to populations of northern fowl mite, Ornithonyssus sylviarum (Canestrini and Fanzago). Poult Sci 59:1742–1744
DeVaney JA, Gyles NR, Lancaster JL (1982) Evaluation of Arkansas Rous-sarcoma regressor and progressor lines and giant jungle fowl for genetic resistance to the northern fowl mite (Acari: Macronyssidae). Poult Sci 61:2327–2330
Dil N, Qureshi MA (2002) Differential expression of CD14 and toll-like receptor-4 imparts LPS-mediated iNOS hypo- and hyper responsiveness in macrophages from varied genetic backgrounds. FASEB J 16:A693–A693
Ditchkoff SS, Hoofer SR, Lochmiller RL, Masters RE, Van den Bussche RA (2005) MHC-DRB evolution provides insight into parasite resistance in white-tailed deer. Southwest Nat 50:57–64 doi:10.1894/0038-4909(2005)050<0057:MEPIIP>2.0.CO;2
Dix MC, Taylor RL (1996) Differential antibody responses in 6.B. Major histocompatibility (B) complex congenic chickens. Poult Sci 75:203–207
Edwards SV, Grahn M, Potts WK (1995) Dynamics of Mhc evolution in birds and crocodilians: amplification of class II genes with degenerate primers. Mol Ecol 4:719–729 doi:10.1111/j.1365-294X.1995.tb00272.x
Edwards SV, Hess CM, Gasper J, Garrigan D (1999) Toward an evolutionary genomics of the avian Mhc. Immunol Rev 167:119–132 doi:10.1111/j.1600-065X.1999.tb01386.x
Eklund J, Loomis E, Abplanalp H (1980) Genetic resistance of white leghorn chickens to infestation by the northern fowl mite, Ornithonyssus sylviarum. Arch Geflugelkd 44:195–199
Froeschke G, Sommer S (2005) MHC class II DRB variability and parasite load in the striped mouse (Rhabdomys pumilio) in the southern Kalahari. Mol Biol Evol 22:1529–1529 doi:10.1093/molbev/msi156
Fulton JE, Juul-Madsen HR, Ashwell CM, McCarron AM, Arthur JA, O’Sullivan NP et al (2006) Molecular genotype identification of the Gallus gallus major histocompatibility complex. Immunogenetics 58:407–421 doi:10.1007/s00251-006-0119-0
Garvin MC, Scheidler LC, Cantor DG, Bell KE (2004) Abundance and temporal distribution of Ornithonyssus sylviarum Canestrini and Fanzago (Acarina: Mesostigmata) in gray catbird (Dumatella carolinensis) nests. J Vector Ecol 29:62–65
Gehad AE, Mashaly MM, Siegel HS, Dunnington EA, Siegel PB (1999) Effect of genetic selection and MHC haplotypes on lymphocyte proliferation and interleukin-2 like activity in chicken lines selected for high and low antibody production against sheep red blood cells. Vet Immunol Immunopathol 68:13–24 doi:10.1016/S0165-2427(99)00008-2
Glatz PC (2000) Beak trimming methods—review. Asian-Australas. J Anim Sci 13:1619–1637
Golemboski KA, Taylor RL, Briles WE, Briles RW, Dietert RR (1995) Inflammatory function of macrophages from chickens with B-recombinant haplotypes. Avian Pathol 24:347–352 doi:10.1080/03079459508419074
Heeb P, Werner I, Kolliker M, Richner H (1998) Benefits of induced host responses against an ectoparasite. Proc R Soc Lond B Biol Sci 265:51–56 doi:doi:10.1098/rspb.1998.0263
Hinkle NC, Hickle LA (1999) California caged layer pest management evaluation. J Appl Poult Res 8:327–338
Hogsette JA, Butler JF, Miller WV, Hall RD (1991) Annotated bibliography of the northern fowl mite, Ornithonyssus sylviarum, (Canestrini and Fanzago) (Acari: Macronyssidae). Misc Publ Entomol Soc Am 76:1–62
IImonen P, Penn DJ, Damjanovich K, Morrison L, Ghotbi L, Potts WK (2007) Major histocompatibility complex heterozygosity reduces fitness in experimentally infected mice. Genetics 176:2501–2508 doi:10.1534/genetics.107.074815
Jarvi SI, Tarr CL, McIntosh CE, Atkinson CT, Fleischer RC (2004) Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Mol Ecol 13:2157–2168 doi:10.1111/j.1365-294X.2004.02228.x
Kaufman J (2000) The simple chicken major histocompatibility complex: life and death in the face of pathogens and vaccines. Philos Trans R Soc Lond B Biol Sci 355:1077–1084 doi:10.1098/rstb.2000.0645
Kaufman J (2008) The avian MHC. In: Davison F, Kaspers B, Schat KA (eds) Avian immunology. Elsevier, Amsterdam, pp 159–181q
Kean RP, Briles WE, Cahaner A, Freeman AE, Lamont SJ (1994) Differences in major histocompatibility complex frequencies after multitrait, divergent selection for immunocompetence. Poult Sci 73:7–17
Kelley J, Walter L, Trowsdale J (2005) Comparative genomics of major histocompatibility complexes. Immunogenetics 56:683–695 doi:10.1007/s00251-004-0717-7
Knee W, Proctor HC (2007) Host records for the northern fowl mite, Ornithonyssus sylviarum (Mesostigmata: Macronyssidae), from birds of North America (Canada, USA and Mexico). J Med Entomol 44:709–713 doi:10.1603/0022-2585(2007)44[709:HRFOSM]2.0.CO;2
Lee KA (2006) Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol 46:1000–1015 doi:10.1093/icb/icl049
Matthysse JG, Jones CJ, Purnasiri A (1974) Development of northern fowl mite populations on chickens, effects on the host, and immunology. Search Agric 4:1–39 Cornell University Experiment Station, Ithaca, NY
Mays JK, Bacon LD, Pandiri AR, Fadly AM (2005) Response of white leghorn chickens of various B haplotypes to infection at hatch with subgroup J Avian Leukosis Virus. Avian Dis 49:214–219 doi:10.1637/7315-120104R
McClelland EE, Damjanovich K, Gardner K, Groesbeck ZJ, Ma MA, Nibley M et al (2004) Infection-dependent phenotypes in MHC-congenic mice are not due to MHC: can we trust congenic animals? BMC Immunol 5:14–21 doi:10.1186/1471-2172-5-14
McConnell SKJ, Dawson DA, Wardle A, Burke T (1999) The isolation and mapping of 19 tetranucleotide microsatellite markers in the chicken. Anim Genet 30:183–189 doi:10.1046/j.1365-2052.1999.00454.x
McCoy KD, Boulinier T, Schjorring S, Michalakis Y (2002) Local adaptation of the ectoparasite Ixodes uriae to its seabird host. Evol Ecol Res 4:441–456
Møller AP (1992) Parasites differentially increase the degree of fluctuating asymmetry in secondary sexual characters. J Evol Biol 5:691–699 doi:10.1046/j.1420-9101.1992.5040691.x
Møller AP (1993) Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol 62:309–322 doi:10.2307/5362
Møller AP (2000) Survival and reproductive rate of mites in relation to resistance of their barn swallow hosts. Oecologia 124:351–357 doi:10.1007/s004420000394
Møller AP, Christe P, Garamszegi LZ (2005) Coevolutionary arms races: increased host immune defense promotes specialization by avian fleas. J Evol Biol 18:46–59 doi:10.1111/j.1420-9101.2004.00774.x
Mullen G, Durden L (2002) Medical and veterinary entomology. Academic, New York
Mullens BA, Hinkle NC, Szijj CE (2000) Monitoring northern fowl mites (Acari: Macronyssidae) in caged laying hens: feasibility of an egg-based sampling system. J Econ Entomol 93:1045–1054
Mullens BA, Owen JP, Kuney DR, Szijj CE, Klingler KA (2008) Temporal changes in distribution, prevalence and intensity of northern fowl mite (Ornithonyssus sylviarum) parasitism in commercial caged laying hens, with a comprehensive economic analysis of parasite impact. Vet Parasitol (submitted)
Nevo E, Beiles A (1992) Selection for class-II MHC heterozygosity by parasites in subterranean mole rats. Experientia 48:512–515 doi:10.1007/BF01928177
Ortego J, Aparicio JM, Calabuig G, Cordero JP (2007) Risk of ectoparasitism and genetic diversity in a wild lesser kestrel population. Mol Ecol 16:3712–3720 doi:10.1111/j.1365-294X.2007.03406.x
Owen JP (2007) Interaction of the host immune response and population dynamics of the northern fowl mite, Ornithonyssus sylviarum, on white leghorn hens. Ph.D. dissertation, pp. 205. University of California, Riverside
Parmentier HK, Baelmans R, Savelkoul HFJ, Dorny P, Demey F, Berkvens D (2004) Serum haemolytic complement activities in 11 different MHC (B) typed chicken lines. Vet Immunol Immunopathol 100:25–32 doi:10.1016/j.vetimm.2004.02.009
Parslow TG, Stites DP, Terr AI, Imboden JB (2001) Medical immunology. McGraw-Hill, New York
Potts WK, Slev PR (1995) Pathogen-based models favoring Mhc genetic diversity. Immunol Rev 143:181–197 doi:10.1111/j.1600-065X.1995.tb00675.x
Puzzi JV, Bacon LD, Dietert RR (1990) B-congenic chickens differ in macrophage inflammatory responses. Vet Immunol Immunopathol 26:13–30 doi:10.1016/0165-2427(90)90129-G
Qureshi MA, Dietert RR, Bacon LD (1988) Chemotactic activity of chicken blood mononuclear leukocytes from 15I5-B-congenic lines to bacterially-derived chemoattractants. Vet Immunol Immunopathol 19:351–360 doi:10.1016/0165-2427(88)90120-1
Randolph SE (1979) Population regulation in ticks: the role of acquired resistance in natural and unnatural hosts. Parasitology 79:141–156
Schad J, Ganzhorn JU, Sommer S (2005) Parasite burden and constitution of major histocompatibility complex in the malagasy mouse lemur, Microcebus murinus. Evolution 59(2):439–450
Sharma JM (1991) Avian cellular immunology. CRC, Boston
Sharma JM (1998) Avian immunology. In: Pastoret P, Griebel HB, Govaerts A (eds) A handbook of vertebrate immunology. Academic, New York
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321 doi:10.1016/0169-5347(96)10039-2
Shiina T, Hosomichi K, Hanzawa K (2006) Comparative genomics of the poultry major histocompatibility complex. Anim Sci J 77:151–162 doi:10.1111/j.1740-0929.2006.00333.x
Shiina T, Briles WE, Goto RM, Hosomichi K, Yanagiya K, Shimizu S et al (2007) Extended gene map reveals tripartite motif, C-type lectin, and Ig superfamily type genes within a subregion of the chicken MHC-B affecting infectious disease. J Immunol 178:7162–7172
Sikes RK, Chamberlain RW (1954) Laboratory observations on 3 species of bird mites. J Parasitol 40:691–697 doi:10.2307/3273713
Stear MJ, Innocent GT, Buitkamp J (2005) The evolution and maintenance of polymorphism in the major histocompatibility complex. Vet Immunol Immunopathol 108:53–57 doi:10.1016/j.vetimm.2005.07.005
Strand T, Westerdahl H, Hoglund J, Alatalo RV, Siitari H (2007) The Mhc class II of the black grouse (Tetrao tetrix) consists of low numbers of B and Y genes with variable diversity and expression. Immunogenetics 59:725–734 doi:10.1007/s00251-007-0234-6
Untalan PM, Pruett JH, Steelman CD (2007) Association of the bovine leukocyte antigen major histocompatibility complex class II DRB3*4401 allele with host resistance to the Lone Star tick, Amblyomma americanum. Vet Parasitol 145:190–195 doi:10.1016/j.vetpar.2006.12.003
Westerdahl H (2007) Passerine MHC: genetic variation and disease resistance in the wild. J Ornithol 148:s469–s477 doi:10.1007/s10336-007-0230-5
Westerdahl H, Waldenstrom J, Hansson B, Hasselquist D, von Schantz T, Bensch S (2005) Associations between malaria and MHC genes in a migratory songbird. Proc R Soc Lond B Biol Sci 272:1511–1518 doi:10.1098/rspb.2005.3113
Whiteman NK, Matson KD, Bollmer JL, Parker PG (2006) Disease ecology in the Galapagos hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc R Soc Lond B Biol Sci 273:797–804 doi:10.1098/rspb.2005.3396
Zhou H, Lamont SJ (2003a) Association of transforming growth factor beta genes with quantitative trait loci for antibody response kinetics in hens. Anim Genet 34:275–282 doi:10.1046/j.1365-2052.2003.01007.x
Zhou H, Lamont SJ (2003b) Chicken MHC class I and II gene effects on antibody response kinetics in adult chickens. Immunogenetics 55:133–140 doi:10.1007/s00251-003-0566-9
Zoorob R, Bernot A, Renoir DM, Choukri F, Auffray C (1993) Chicken major histocompatibility complex class-Ii B-genes—analysis of interallelic and interlocus sequence variance. Eur J Immunol 23:1139–1145 doi:10.1002/eji.1830230524
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22 doi:10.1086/342131
Acknowledgements
This work was supported by a grant from the US Department of Agriculture, National Research Initiative (USDA Cooperative State Research, Education and Extension Service, grant number #2005-35302-16341) and USDA-CSREES Multistate Research funds (Delany—CA-D*-ASC-7281-RR) and special facilities support from the UC Davis College of Agricultural and Environmental Sciences. Dr. Carol Cardona (University of Californa, Davis) was very helpful in initial discussions regarding the potential role of MHC variation in mite resistance. We would like to thank Dr. Jackie Pisenti and Tom O’Hare for their help in breeding and hatching the congenic birds, as well as Kathryn Haith and Martie Pastor for general animal care. We would also like to thank Dr. Janet Fulton (Hy-Line International) for guidance on MHC genotyping and comments on the manuscript. Kimberly Klingler provided invaluable support for the genotyping. Finally, we would like to thank Dr. Wayne Potts (University of Utah) and three anonymous reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owen, J.P., Delany, M.E. & Mullens, B.A. MHC haplotype involvement in avian resistance to an ectoparasite. Immunogenetics 60, 621–631 (2008). https://doi.org/10.1007/s00251-008-0314-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-008-0314-2