Abstract
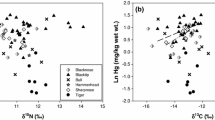
Few published studies have examined whether the elevated concentrations of the nonessential toxic metal mercury (Hg) often observed in shark muscle also occur in the shark brain or whether Hg accumulation affects shark neurophysiology. Therefore, this study examined accumulation and distribution of Hg in the shark brain, as well as effects of Hg on oxidative stress in the shark central nervous system, with particular focus on the Atlantic sharpnose shark (Rhizoprionodon terraenovae). Sharks were collected along the southeastern U.S. coast throughout most of this species’ U.S. geographical range. Total Hg (THg) concentrations were measured in and compared between shark muscle and brain, whereas known biomarkers of Hg-induced neurological effects, including glutathione depletion, lipid peroxidation, and concentrations of a protein marker of glial cell damage (S100b), were measured in shark cerebrospinal fluid. Brain THg concentrations were correlated with muscle THg levels but were significantly lower and did not exceed most published thresholds for neurological effects, suggesting limited potential for detrimental responses. Biomarker concentrations supported this premise, because these data were not correlated with brain THg levels. Hg speciation also was examined. Unlike muscle, methylmercury (MeHg) did not comprise a high percentage of THg in the brain, suggesting that differential uptake or loss of organic and inorganic Hg and/or demethylation of MeHg may occur in this organ. Although Hg accumulation in the shark brain generally fell below toxicity thresholds, higher THg levels were measured in the shark forebrain compared with the midbrain and hindbrain. Therefore, there is potential for selective effects on certain aspects of shark neurophysiology if brain Hg accumulation is increased.










Similar content being viewed by others
References
Adams DH, McMichael RH (1999) Mercury levels in four species of sharks from the Atlantic coast of Florida. Fish Bull 97:372–379
Adams DH, McMichael RH, Henderson GE (2003) Mercury levels in marine and estuarine fishes of Florida 1989–2001, 2nd edn revised. Florida fish and wildlife conservation commission, Florida Marine Research InstituteTechnical Report TR-9
Adams DH, Sonne C, Basu N, Dietz R, Nam DH, Leifsson PS, Jensen AL (2010) Mercury contamination in spotted seatrout, Cynoscion nebulosus: an assessment of liver, kidney blood, and nervous system health. Sci Total Environ 408:5808–5816
Bergés-Tiznado ME, Marquez-Farias F, Lara-Mendoza RE, Torres-Rojas YE, Galvan-Magana F, Bojorquez-Leyva H, Paez-Osuna F (2015) Mercury and selenium in muscle and target organs of scalloped hammerhead sharks Sphyrna lewini of the SE gulf of california: dietary intake, molar ratios, loads, and human health risks. Arch Environ Contam Toxicol 69:440–452. https://doi.org/10.1007/s00244-015-0226-8
Berlin M, Grant CA, Hellberg J, Hellstrom J, Schultz A (1975) Neurotoxicity of methylmercury in squirrel monkeys. Cerebral cortical pathology, interference with scotopic vision, and changes in operant behavior. Arch Environ Health 30:340–348. https://doi.org/10.1080/00039896.1975.10666717
Berntssen MH, Aatland A, Handy RD (2003) Chronic dietary mercury exposure causes oxidative stress, brain lesions, and altered behaviour in Atlantic salmon (Salmo salar) parr. Aquat Toxicol 65:55–72. https://doi.org/10.1016/S0166-445X(03)00104-8
Bethea DM, Carlson JK, Buckel JA, Satterwhite M (2006) Ontogenetic and site-related trends in the diet of the Atlantic sharpnose shark Rhizoprionodon terraenovae from the northeast Gulf of Mexico. Bull Mar Sci 78:287–307
Bridges CC, Zalups RK (2010) Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev 13:385–410
Chan MC, Bautista E, Alvarado-Cruz I, Quintanilla-Vega B, Segovia J (2017) Inorganic mercury prevents the differentiation of SH-SY5Y cells: amyloid precursor protein, microtubule associated proteins and ROS as potential targets. J Trace Elem Med Biol 41:119–128l. https://doi.org/10.1016/j.jtemb.2017.02.002
Charbonneau SM, Munro IC, Nera EA, Armstrong FA, Willes RF, Bryce F, Nelson RF (1976) Chronic toxicity of methylmercury in the adult cat Interim report. Toxicology 5:337–349. https://doi.org/10.1016/0300-483X(76)90052-4
Chiba A (2000) S-100 protein-immunoreactive structures in the brains of the elasmobranchs Scyliorhinus torazame and Mustelus manazo. Neurosci Lett 279:65–68
Depew DC, Basu N, Burgess NM, Campbell LM, Devlin EW, Drevnick PE, Hammerschmidt CR, Murphy CA, Sandheinrich MB, Wiener JG (2012) Toxicity of dietary methylmercury to fish: derivation of ecologically meaningful threshold concentrations. Environ Toxicol Chem 31:1536–1547. https://doi.org/10.1002/etc.1859
Dietz R, Sonne C, Basu N, Braune B, O’Hara T, Letcher RJ, Scheuhammer T, Andersen M, Andreasen C, Andriashek D, Asmund G, Aubail A, Baagøe H, Born EW, Chan HM, Derocher AE, Grandjean P, Knott K, Kirkegaard M, Krey A, Lunn N, Messier F, Obbard M, Olsen MT, Ostertag S, Peacock E, Renzoni A, Rigét FF, Skaare JU, Stern G, Stirling I, Taylor M, Wiig Ø, Wilson S, Aars J (2013) What are the toxicological effects of mercury in Arctic biota? Sci Total Environ 443:775–790
Drymon JM, Powers SP, Dindo J, Dzwonkowsi B, Henwood T (2010) Distribution of sharks across a continental shelf in the northern Gulf of Mexico. Mar Coast Fish 2:440–450
Drymon JM, Powers SP, Carmichael RH (2012) Trophic plasticity in the Atlantic sharpnose shark (Rhizoprionodon terraenovae) from the north central Gulf of Mexico. Environ Biol Fish 95:21–35. https://doi.org/10.1007/s10641-011-9922-z
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JB, Marquis RJ (2011) Trophic downgrading of planet Earth. Science 333:301–306. https://doi.org/10.1126/science.1205106
Evers DC, Hammerschlag N, Die D (2008) Mercury levels in Florida sharks: interim report. Report BRI 2008-02:1-16. Gorham, Maine
Farina M, Cereser V, Portela LV, Mendez A, Porciúncula LO, Fornaguera J, Gonçalves CA, Wofchuk ST, Rocha JBT, Souza DO (2005) Methylmercury increases S100B content in rat cerebrospinal fluid. Environ Toxicol Pharmacol 19:249–253. https://doi.org/10.1016/j.etap.2004.07.008
Farina M, Rocha JB, Aschner M (2011) Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89:555–563. https://doi.org/10.1016/j.lfs.2011.05.019
Ferretti P (2011) Is there a relationship between adult neurogenesis and neuron generation following injury across evolution? Eur J Neurosci 34:951–962. https://doi.org/10.1111/j.1460-9568.2011.07833.x
Finger J, Kubke M, Wild J, Montgomery J (2008) An examination of the extent of adult neurogenesis in the carpet shark (Cephaloscyllium isabellum). Paper presented at the 26th international australasian winter conference on brain research
Finlay BL, Darlington RB, Nicastro N (2001) Developmental structure in brain evolution. Behav Brain Sci 24:263–278. https://doi.org/10.1017/S0140525X01003958
Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Pomblum SCG, Moro ÂM, Bohrer D, Bairros AV, Dafre AL, Santos AR (2006) Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res 102:22–28. https://doi.org/10.1016/j.envres.2006.02.003
Gelsleichter J, Walker CJ (2010) Pollutant exposure and effects in sharks and their relatives. In: Carrier JC, Musick JA, Heithaus MR (eds) Sharks and their relatives II: biodiveristy, adaptive physiology, and conservation. CRC Press, Boca Raton, pp 491–540
Gelsleichter J, Musick JA, Nichols S (1999) Food habits of the smooth dogfish, Mustelus canis, dusky shark, Carcharhinus obscurus, Atlantic sharpnose shark, Rhizoprionodon terraenovae, and the sand tiger, Carcharias taurus, from the northwest Atlantic Ocean. Environ Biol Fish 54:205–217. https://doi.org/10.1023/A:1007527111292
Greco A, Minghetti L, Sette G, Fieschi C, Levi G (1999) Cerebrospinal fluid isoprostane shows oxidative stress in patients with multiple sclerosis. Neurology 53:1876-1876. https://doi.org/10.1212/WNL.53.8.1876
Grippo MA, Heath AG (2003) The effect of mercury on the feeding behavior of fathead minnows (Pimephales promelas). Ecotoxicol Environ Saf 55:187–198. https://doi.org/10.1016/S0147-6513(02)00071-4
Harris R, Pollman C, Landing W, Evans D, Axelrad D, Hutchinson D, Morey SL, Rumbold D, Dukhovskoy D, Adams DH, Vijayaraghavan K, Holmes C, Atkinson RD, Myers T, Sunderland E (2012) Mercury in the Gulf of Mexico: sources to receptors. Environ Res 119:42–52
Hoffman DJ, Eagles-Smith CA, Ackerman JT, Adelsbach TL, Stebbins KR (2011) Oxidative stress response of Forster’s terns (Sterna forsteri) and Caspian terns (Hydroprogne caspia) to mercury and selenium bioaccumulation in liver, kidney, and brain. Environ Toxicol Chem 30:920–929
Kaur P, Aschner M, Syversen T (2006) Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. NeuroToxicology 27:492–500. https://doi.org/10.1016/j.neuro.2006.01.010
Krey A, Kwan M, Chan HM (2012) Mercury speciation in brain tissue of polar bears (Ursus maritimus) from the Canadian Arctic. Environ Res 114:24–30. https://doi.org/10.1016/j.envres.2012.01.006
Krey A, Ostertag SK, Chan HM (2015) Assessment of neurotoxic effects of mercury in beluga whales (Delphinapterus leucas), ringed seals (Pusa hispida), and polar bears (Ursus maritimus) from the Canadian Arctic. Sci Total Environ 509–510:237–247. https://doi.org/10.1016/j.scitotenv.2014.05.134
Loefer JK, Sedberry GR (2003) Life history of the Atlantic sharpnose shark (Rhizoprionodon terraenovae) (Richardson, 1836) off the southeastern United States. Fish Bull 101:75–88
Lohren H, Bornhorst J, Fitkau R, Pohl G, Galla HJ, Schwerdtle T (2016) Effects on and transfer across the blood–brain barrier in vitro-comparison of organic and inorganic mercury species. BMC Pharmacol Toxicol 17:63
Lyons K, Carlisle A, Preti A, Mull C, Blasius M, O’Sullivan J, Winkler C, Lowe CG (2013) Effects of trophic ecology and habitat use on maternal transfer of contaminants in four species of young of the year lamniform sharks. Mar Environ Res 90:27–38. https://doi.org/10.1016/j.marenvres.2013.05.009
Mahboob M, Shireen KF, Atkinson A, Khan AT (2001) Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J Environ Sci Health B 36:687–697. https://doi.org/10.1081/PFC-100106195
Mailloux RJ, Yumvihoze E, Chan HM (2015) Superoxide anion radical (O2−) degrades methylmercury to inorganic mercury in human astrocytoma cell line (CCF-STTG1). Chem Biol Interact 239:46–55. https://doi.org/10.1016/j.cbi.2015.06.028
Mickle A (2016) Trophic ecology and bioaccumulation of mercury in the three hagfish (Myxinidae) species from the Gulf of Mexico. Thesis. The Florida State University
Mieiro CL, Ahmad I, Pereira ME, Duarte AC, Pacheco M (2010) Antioxidant system breakdown in brain of feral golden grey mullet (Liza aurata) as an effect of mercury exposure. Ecotoxicology 19:1034–1045
Mieiro CL, Pereira ME, Duarte AC, Pacheco M (2011) Brain as a critical target of mercury in environmentally exposed fish (Dicentrarchus labrax)—bioaccumulation and oxidative stress profiles. Aquat Toxicol 103:233–240. https://doi.org/10.1016/j.aquatox.2011.03.006
Mull CG, Yopak KE, Dulvy NK (2011) Does more maternal investment mean a larger brain? Evolutionary relationships between reproductive mode and brain size in chondrichthyans. Mar Freshw Res 62:567–575. https://doi.org/10.1071/MF10145
Nam DH, Adams DH, Reyier EA, Basu N (2011a) Mercury and selenium levels in lemon sharks (Negaprion brevirostris) in relation to a harmful red tide event. Environ Monit Assess 176(1–4):549–559. https://doi.org/10.1007/s10661-010-1603-4
Nam D, Head J, Adams D, Evers DC, Carvan M, Goetz FW, Murphy C, Basu N (2011b) Neuroendocrine effects of mercury in several fish species. Conference Abstract: NASCE 2011: the inaugural meeting of the North American society of comprehensive endocrinology. https://doi.org/10.3389/conf.fendo.2011.04.00089
Newman MC, Xu X, Cotton CF, Tom KR (2011) High mercury concentrations reflect trophic ecology of three deep-water chondrichthyans. Arch Environ Contam Toxicol 60:618–625. https://doi.org/10.1007/s00244-010-9584-4
Northcutt RG (1978) Brain organization in the cartilaginous fishes. Sensory biology of sharks, skates, and rays. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates, and rays. Office of Naval Research, Arlington, pp 117–193
Parsons GR, Hoffmayer ER (2005) Seasonal changes in the distribution and relative abundance of the Atlantic sharpnose shark Rhizoprionodon terraenovae in the north central Gulf of Mexico. Copeia 2005:914–920
Pereira P, Raimundo J, Araújo O, Canário J, Almeida A, Pacheco M (2014) Fish eyes and brain as primary targets for mercury accumulation—a new insight on environmental risk assessment. Sci Total Environ 494–495:290–298
Pereira P, Puga S, Cardoso V, Pinto-Ribeiro F, Raimundo J, Barata M, Pousão-Ferreira P, Pacheco M, Almeida A (2016) Inorganic mercury accumulation in brain following waterborne exposure elicits a deficit on the number of brain cells and impairs swimming behavior in fish (white seabream-Diplodus sargus). Aquat Toxicol 170:400–412. https://doi.org/10.1016/j.aquatox.2015.11.031
Pethybridge H, Cossa D, Butler EC (2010) Mercury in 16 demersal sharks from southeast Australia: biotic and abiotic sources of variation and consumer health implications. Mar Environ Res 69:18–26. https://doi.org/10.1016/j.marenvres.2009.07.006
Puga S, Pereira P, Pinto-Ribeiro F, O’Driscoll NJ, Mann E, Barata M, Pousão-Ferreira P, Canário J, Almeida A, Pacheco M (2016) Unveiling the neurotoxicity of methylmercury in fish (Diplodus sargus) through a regional morphometric analysis of brain and swimming behavior assessment. Aquat Toxicol 180:320–333. https://doi.org/10.1016/j.aquatox.2016.10.014
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47:74–83
Rodgers DW, Beamish FWH (1982) Dynamics of dietary methylmercury in rainbow trout, Salmo gairdneri. Aquat Toxicol 2:271–290
Rouleau C, Borg-Neczak K, Gottofrey J, Tjälve H (1999) Accumulation of waterborne mercury(II) in specific areas of fish brain. Environ Sci Technol 33:3384–3389
Rumbold D, Wasno R, Hammerschlag N, Volety A (2014) Mercury accumulation in sharks from the coastal waters of southwest Florida. Arch Environ Contam Toxicol 67:402–412. https://doi.org/10.1007/s00244-014-0050-6
Sandheinrich MB, Miller KM (2006) Effects of dietary methylmercury on reproductive behavior of fathead minnows (Pimephales promelas). Environ Toxicol Chem 25:3053–3057. https://doi.org/10.1897/05-641R.1
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. https://doi.org/10.1016/j.aquatox.2004.03.016
Shapiro AM, Chan HM (2008) Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere 74:112–118. https://doi.org/10.1016/j.chemosphere.2008.09.019
Shaw BP, Sahu A, Panigrahi AK (1990) Comparative toxicity of an effluent from a chlor-alkali industry and HgCl2. Bull Environ Contam Toxicol 45:280–287
Storelli MM, Giacominelli-Stuffler R, Marcotrigiano G (2002) Mercury accumulation and speciation in muscle tissue of different species of sharks from Mediterranean Sea, Italy. Bull Environ Contam Toxicol 68:201–210. https://doi.org/10.1007/s001280239
Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M (2008) Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227:147–154. https://doi.org/10.1016/j.taap.2007.10.010
Suzuki T (1979) Dose-effect and dose-response relationships of mercury and its derivatives. In: Nriagu, JO (ed) The biogeochemistry of mercury in the environment. Elsevier/North-Holland Press, New York, pp 399–431
U.S. EPA (2001) Water quality criteria for the protection of human health: methylmercury, EPA-823- R-01- 001. Office of Sciences and Technology, Office of Water, Washington
U.S. EPA (2007) Method 7473: mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. US Environmental Protection Agency, Washington
Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol 134:273–284. https://doi.org/10.1006/taap.1995.1193
Vicente É, Boer M, Leite M, Silva M, Tramontina F, Porciúncula L, Dalmaz C, Gonçalves CA (2004) Cerebrospinal fluid S100B increases reversibly in neonates of methyl mercury-intoxicated pregnant rats. NeuroToxicol 25(5):771–777. https://doi.org/10.1016/j.neuro.2004.03.001
Webber HM, Haines TA (2003) Mercury effects on predator avoidance behavior of a forage fish, golden shiner (Notemigonus crysoleucas). Environ Toxicol Chem 22:1556–1561
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA, Cairns J (eds) Handbook of ecotoxicology, vol 2, 2nd edn. CRC Press, Boca Raton, pp 409–463
Yopak KE (2012) Neuroecology of cartilaginous fishes: the functional implications of brain scaling. J Fish Biol 80:1968–2023. https://doi.org/10.1111/j.1095-8649.2012.03254.x
Yopak KE, Lisney TJ (2012) Allometric scaling of the optic tectum in cartilaginous fishes. Brain Behave Evol 80:108–126. https://doi.org/10.1159/000339875
Yopak KE, Montgomery JC (2008) Brain organization and specialization in deep-sea chondrichthyans. Brain Behav Evol 71:287–304. https://doi.org/10.1159/000127048
Yopak KE, Lisney TJ, Collin SP, Montgomery JC (2007) Variation in brain organization and cerebellar foliation in chondrichthyans: sharks and holocephalans. Brain Behav Evol 69:280–300. https://doi.org/10.1159/000100037
Yopak KE, Lisney TJ, Darlington RB, Collin SP, Montgomery JC, Finlay BL (2010) A conserved pattern of brain scaling from sharks to primates. Proc Natl Acad Sci USA 107(29):12946–12951. https://doi.org/10.1073/pnas.1002195107
Yoshida M, Shimada E, Arai F, Yamamura Y (1980) The relation between mercury levels in brain and blood or cerebrospinal fluid (CSF) after mercury exposure. J Toxicol Sci 5:243–250. https://doi.org/10.2131/jts.5.243
Zheng W, Aschner M, Ghersi-Egea JF (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol 192:1–11. https://doi.org/10.1016/S0041-008X(03)00251-5
Acknowledgements
The authors acknowledge the following individuals for their contributions to this study: K. Yopak, J. Ochrietor, C. Bangley, C. Belcher, M. Benavides, D. Bethea, C. Carpenter, J. Davis, C. Dean, T. Driggers, M. Drymon, B. Falterman, B. Frazier, M. Gonzalez De Acevedo, J. Gregg, R.D. Grubbs, J. Hendon, J. Imhoff, J. Kohn, R. Latour, D. McDowell, C. Morgan, K. Mowle, C. Peterson, J. Russo, C. Shields, and J. Whalen. This research was supported by the University of North Florida. Additional funding for animal collections was provided through contracts from the National Oceanic and Atmospheric Association, National Marine Fisheries Service (NOAA–NMFS), Cooperative Atlantic States Shark Pupping and Nursery Survey Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehnert-Russo, S.L., Gelsleichter, J. Mercury Accumulation and Effects in the Brain of the Atlantic Sharpnose Shark (Rhizoprionodon terraenovae). Arch Environ Contam Toxicol 78, 267–283 (2020). https://doi.org/10.1007/s00244-019-00691-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00691-0