Abstract
Purpose
Ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, is predominantly metabolized by cytochrome P450 3A and both the parent compound and its active metabolite AR-C124910XX are substrates of P-glycoprotein. Rifampicin was used to assess the effects of CYP3A and P-glycoprotein induction on the single-dose pharmacokinetics and pharmacodynamics of ticagrelor.
Methods
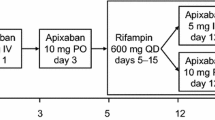
Healthy volunteers received a single 180 mg oral dose of ticagrelor on days 1 and 15, and a once-daily 600 mg dose of rifampicin on days 4–17. Ticagrelor and AR-C124910XX plasma concentrations were quantified for pharmacokinetic analysis (n = 14); inhibition of platelet aggregation (IPA) was also assessed (n = 14).
Results
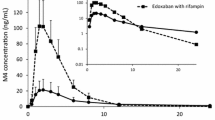
Compared with administration of ticagrelor alone, co-administration of ticagrelor and rifampicin significantly decreased the maximum plasma concentration (Cmax) of ticagrelor from 1091 to 297.8 ng/ml, area under the plasma concentration-time curve from time zero to infinity (AUC) of ticagrelor from 6225 to 864.0 ng.h/ml, and also decreased plasma half-life of ticagrelor from 8.4 to 2.8 h; reductions of 73 %, 86 % and 67 % respectively. With rifampicin, AR-C124910XX Cmax was unaffected, AUC was significantly decreased by 46 %, and metabolite to parent ratio for AUC increased fourfold. Although maximal IPA was unaffected, offset of ticagrelor-mediated IPA was more rapid in the presence of rifampicin; a significant reduction (27 %) in the area under the effect curve between 0 and 24 h was observed following co-administration with rifampicin.
Conclusion
Co-administration with rifampicin reduced ticagrelor exposure and resulted in a more rapid offset of ticagrelor-mediated IPA. Co-administration of strong CYP3A/P-glycoprotein inducers with ticagrelor should be discouraged.



Similar content being viewed by others
References
Husted S, van Giezen JJ (2009) Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther 27:259–274
Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G (2006) Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 27:1038–1047
Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P, Cannon CP (2007) Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 50:1852–1856
Brilique, summary of product characteristics, 2010. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001241/WC500100494.pdf. Accessed 30 January 2012
Brilinta™, US full prescribing information, July 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022433s000lbl.pdf. Accessed 30 January 2012
Butler K, Teng R (2010) Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 70:65–77
Teng R, Butler K (2008) AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect in healthy subjects. Can J Clin Pharmacol 15:e426
Teng R, Butler K (2010) Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjects. Eur J Clin Pharmacol 66:487–496
Savi P, Combalbert J, Gaich C, Rouchon MC, Maffrand JP, Berger Y, Herbert JM (1994) The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. J Thromb Haemost 72:313–317
van Giezen JJJ, Nilsson L, Berntsson P, Zachrisson H, Bjorkman J-A (2009) Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost 7:556–565
Hagihara K, Kazui M, Kurihara A, Iwabuchi H, Ishikawa M, Kobayashi H, Tanaka N, Okazaki O, Farid NA, Ikeda T (2010) Biotransformation of prasugrel, a novel thienopyridine antiplatelet agent, to the pharmacologically active metabolite. Drug Metab Dispos 38:898–904
Teng R, Oliver S, Hayes MA, Butler K (2010) Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos 38:1514–1521
van Giezen JJJ (2008) Optimizing platelet inhibition. Eur Heart J 10(suppl D):D23–D29
Zhou D, Andersson TB, Grimm SW (2011) In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome p450 reaction phenotyping, inhibition, induction and differential kinetics. Drug Metab Dispos 39:703–710
Sousa M, Pozniak A, Boffito M (2008) Pharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugs. J Antimicrob Chemother 62:872–878
Backman JT, Granfors MT, Neuvonen PJ (2006) Rifampin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol 62:451–461
Raybon JJ, Pray D, Morgan DG, Zoeckler M, Zheng M, Sinz M, Kim S (2011) Pharmacokinetic-pharmacodynamic modeling of rifampicin-mediated Cyp3a11 induction in steroid and xenobiotic X receptor humanized mice. J Pharmacol Exp Ther 337:75–82
Daujat M, Clair P, Astier C, Pineau T, Yerle M, Gellin J, Maurel P (1991) Induction, regulation and messenger half-life of cytochromes P450 IA1, IA2 and IIIA6 in primary cultures of rabbit hepatocytes. CYP 1A1, 1A2 and 3A6 chromosome location in the rabbit and evidence that post-transcriptional control of gene IA2 does not involve mRNA stabilization. Eur J Biochem 200:501–510
Food and Drug Administration. Guidance for industry: Drug interaction studies – study design, data analysis and implications for dosing and labeling. Sep 2006. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf. Accessed 30 January 2012
Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT (2003) Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 42:819–850
Reitman ML, Chu X, Cai X, Yabut J, Venkatasubramanian R, Zajic S, Stone JA, Ding Y, Witter R, Gibson C, Roupe K, Evers R, Wagner JA, Stoch A (2011) Rifampin’s acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 89:234–242
Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B (2007) ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction. Circulation 116:e148–e304
Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W, Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology (2007) Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 28:1598–1660
Sillén H, Cook M, Davis P (2010) Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878:2299–2306
Meen Ø, Brosstad F, Bjørnsen S, Pedersen TM, Erikssen G (2009) Variability in aggregometry response before and after initiation of clopidogrel therapy. Scand J Clin Lab Invest 69(6):673–679
Labarthe J, Théroux P, Angioï M, Ghitescu M (2005) Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol 46:638–645
Kajosaari LI, Laitila J, Neuvonen PJ, Backman JT (2005) Metabolism of repaglinide by CYP2C8 and CYP3A4 in vitro: effect of fibrates and rifampicin. Basic Clin Pharmacol Toxicol 97:249–256
Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, Storey RF (2007) Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 50:1844–1851
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361:1045–1057
Acknowledgments
Medical writing support was provided by David Evans at Gardiner-Caldwell Communications, and was funded by AstraZeneca.
Conflict of interest
All authors are employees of, and this study was funded by, AstraZeneca LP (Wilmington, Delaware, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teng, R., Mitchell, P. & Butler, K. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of ticagrelor in healthy subjects. Eur J Clin Pharmacol 69, 877–883 (2013). https://doi.org/10.1007/s00228-012-1436-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1436-x