Abstract
Purpose
Although the prevalence of drug–drug interactions (DDIs) in elderly outpatients is high, many potential DDIs do not have any actual clinical effect, and data on the occurrence of DDI-related adverse drug reactions (ADRs) in elderly outpatients are scarce. This study aimed to determine the incidence and characteristics of DDI-related ADRs among elderly outpatients as well as the factors associated with these reactions.
Methods
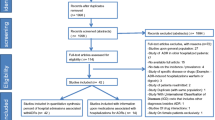
A prospective cohort study was conducted between 1 November 2010 and 31 November 2011 in the primary public health system of the Ourinhos micro-region, Brazil. Patients aged ≥60 years with at least one potential DDI were eligible for inclusion. Causality, severity, and preventability of the DDI-related ADRs were assessed independently by four clinicians using validated methods; data were analysed using descriptive analysis and multiple logistic regression.
Results
A total of 433 patients completed the study. The incidence of DDI-related ADRs was 6 % (n = 30). Warfarin was the most commonly involved drug (37 % cases), followed by acetylsalicylic acid (17 %), digoxin (17 %), and spironolactone (17 %). Gastrointestinal bleeding occurred in 37 % of the DDI-related ADR cases, followed by hyperkalemia (17 %) and myopathy (13 %). The multiple logistic regression showed that age ≥80 years [odds ratio (OR) 4.4; 95 % confidence interval (CI) 3.0–6.1, p < 0.01], a Charlson comorbidity index ≥4 (OR 1.3; 95 % CI 1.1–1.8, p < 0.01), consumption of five or more drugs (OR 2.7; 95 % CI 1.9–3.1, p < 0.01), and the use of warfarin (OR 1.7; 95 % CI1.1–1.9, p < 0.01) were associated with the occurrence of DDI-related ADRs. With regard to severity, approximately 37 % of the DDI-related ADRs detected in our cohort necessitated hospital admission. All DDI-related ADRs could have been avoided (87 % were ameliorable and 13 % were preventable). The incidence of ADRs not related to DDIs was 10 % (n = 44).
Conclusions
The incidence of DDI-related ADRs in elderly outpatients is high; most events presented important clinical consequences and were preventable or ameliorable.

Similar content being viewed by others
References
Hansten PD, Horn JR (2009) Drug interactions analysis and management. Facts & Comparisons. Lippincott Williams & Wilkins, Philadelphia
Cruciol-Souza JM, Thomson JC (2006) Prevalence of potential drug–drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci 9:427–433
Obreli-Neto PR, Vieira JC, Teixeira DRA, Silva FP, Gaeti WP, Cuman RKN (2011) Potential risks in drug prescriptions to elderly: a cross-sectional study in the public primary health care system of Ourinhos micro-region, Brazil. Acta Farm Bonaerense 30:629–635
Björkman IK, Fastbom J, Schmidt IK, Bernsten CB, Pharmaceutical Care of the Elderly in Europe Research (PEER) Group (2002) Drug-drug interactions in the elderly. Ann Pharmacother 36:1675–1681. doi:10.1345/aph.1A484
Tulner LR, Frankfort SV, Gijsen GJ, van Campen JP, Koks CH, Beijnen JH (2008) Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging 25:343–355
Hamilton RA, Briceland LL, Andritz MH (1998) Frequency of hospitalization after exposure to known drug–drug interactions in a Medicaid population. Pharmacotherapy 18:1112–1120
Shad MU, Marsh C, Preskorn SH (2001) The economic consequences of a drug–drug interaction. J Clin Psychopharmacol 21:119–120
Grymonpre RE, Mitenko PA, Sitar DS, Aoki FY, Montgomery PR (1998) Drug-associated hospital admissions in older medical patients. J Am Geriatr Soc 36:1092–1098
Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57:6–14. doi:10.1046/j.1365-2125.2003.02007.x
Mallet L, Spinewine A, Huang A (2007) The challenge of managing drug interactions in elderly people. Lancet 370:185–191. doi:10.1016/S0140-6736(07)61092-7
Cusack BJ (2004) Pharmacokinetics in older persons. Am J Geriatr Pharmacother 2:274–302. doi:10.1016/j.amjopharm.2004.12.005
Turnheim K (2003) When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 38:843–853. doi:10.1016/S0531-5565(03)00133-5
Noble RE (2003) Drug therapy in the elderly. Metabolism 52:27–30. doi:10.1016/S0026-0495(03)00298-114
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M (2009) Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One 4:e4439. doi:10.1371/journal.pone.0004439
Reis AM, Cassiani SH (2011) Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol 67:625–632. doi:10.1007/s00228-010-0987-y
República Federativa do Brasil (1990) Lei n. 8080, 19 de setembro de 1990. Dispõe sobre as condições para a promoção, proteção e recuperação da saúde, a organização e o funcionamento dos serviços correspondentes e dá outras providências. http://portal.in.gov.br/. Accessed 09 October 2010
República Federativa do Brasil (1990) Lei n. 8142, de 28 de dezembro de 1990. Dispõe sobre a participação da comunidade na gestão do Sistema Único de Saúde (SUS) e sobre as transferências intergovernamentais de recursos financeiros na área da saúde e dá outras providências. http://portal.in.gov.br/. Accessed 09 October 2010
United Nations Programme on Ageing (2010) The Ageing of the World's Population. http://www.un.org/ageing/popageing.html. Accessed 10 October 2010
World Health Organization (1984) The uses of epidemiology in the study of the elderly. World Health Organization, Geneva
DrugDigest (2010) Check interactions. http://www.drugdigest.org/wps/portal/ddigest. Accessed 12 October 2010
Drugs (2010) Drug interaction checker. http://www.drugd.com/drug_interactions.html. Accessed 12 October 2010
Medscape (2010) Drug information. http://www.medscape.com/druginfo/druginterchecker. Accessed 12 October 2010
Micromedex (2010) Micromedex healthcare series. http://www.periodicos.capes.gov.br/. Accessed 13 October 2010
Vonbach P, Dubied A, Krähenbühl S, Beer JH (2008) Evaluation of frequently used drug interaction screening programs. Pharm World Sci 30:367–374. doi:10.1007/s11096-008-9191-x
World Health Organization Collaborating Centre for Drug Statistics Methodology (2010) ATC⁄DDD Index. http://www.whocc.no/atcddd/. Accessed 15 October 2010
Karch FE, Lasagna L (1977) Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther 21:247–254
Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR (1979) An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA 242:623–632. doi:10.1001/jama.1979.03300070019017
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245. doi:10.1038/clpt.1981.154
Macedo AF, Marques FB, Ribeiro CF, Teixeira F (2003) Causality assessment of adverse drug reactions: comparison of the results obtained from published decisional algorithms and from the evaluations of an expert panel, according to different levels of imputability. J Clin Pharm Ther 28:137–143. doi:10.1046/j.1365-2710.2003.00475.x
Hartwig SC, Siegel J, Schneider PJ (1992) Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 49:2229–2232
Davies EC, Green CF, Mottram DR, Pirmohamed M (2006) Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther 31:335–341. doi:10.1111/j.1365-2710.2006.00744.x
Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW (2003) Adverse drug events in ambulatory care. N Engl J Med 348:1556–1564. doi:10.1056/NEJMsa020703
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, Motola D, Tuccori M, Conforti A (2010) Identifying adverse drug reactions associated with drug–drug interactions: data mining of a spontaneous reporting database in Italy. Drug Saf 33:667–675. doi:10.2165/11534400-000000000-00000
Strandell J, Wahlin S (2011) Pharmacodynamic and pharmacokinetic drug interactions reported to VigiBase, the WHO global individual case safety report database. Eur J Clin Pharmacol 67:633–641. doi:10.1007/s00228-010-0979-y
Hohl CM, Dankoff J, Colacone A, Afilalo M (2001) Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med 38:666–671. doi:10.1067/mem.2001.119456
Pirmohamed M, James S, Meakin S, Green C, Scot AK, Walley TL, Farrar K, Park BK, Breckenridge AM (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. Br Med J 329:15–19. doi:10.1136/bmj.329.7456.15
Shorr RI, Ray WA, Daugherty JR, Griffin MR (1993) Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants. Arch Intern Med 153:1665–1670
Hernández-Díaz S, Rodríguez LA (2000) Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med 160:2093–2099
Perazella MA, Mahnensmith RL (1997) Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med 12:646–656. doi:10.1046/j.1525-1497.1997.07128.x
Schepkens H, Vanholder R, Billiouw JM, Lameire N (2001) Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med 110:438–441. doi:10.1016/S0002-9343(01)00642-8
Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD (2010) Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 33:171–187. doi:10.2165/11319380-000000000-00000
Smith TW, Antman EM, Friedman PL, Blatt CM, Marsh JD (1984) Digitalis glycosides: mechanisms and manifestations of toxicity. Part I. Prog Cardiovasc Dis 26:413–458
Smith TW, Antman EM, Friedman PL, Blatt CM, Marsh JD (1984) Digitalis glycosides: mechanisms and manifestations of toxicity. Part II. Prog Cardiovasc Dis 26:495–540
Sandson N (2005) Drug-drug interactions: the silent epidemic. Psychiatr Serv 56:22–24. doi:10.1176/appi.ps.56.1.22
Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, Lipton RB (2003) Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc 44:136–141
Guidoni CM, Baldoni AO, Obreli-Neto PR, Pereira LRL (2011) Fontes de informações sobre interações medicamentosas: Há concordância entre elas? Rev Univ Vale Rio Verde 9:84–91. doi:10.5892/ruvrv.2011.92.8491
Vitry AI (2007) Comparative assessment of four drug interaction compendia. Br J Clin Pharmacol 63:709–714. doi:10.1111/j.1365-2125.2006.02809.x
Caccia S, Garattini S, Pasina L, Nobili A (2009) Predicting the clinical relevance of drug interactions from pre-approval studies. Drug Saf 32:1017–1039. doi:10.2165/11316630-000000000-00000
Acknowledgments
We acknowledge the clinical pharmacists and physicians involved in the study. This study was supported by Fundação de Apoio ao Desenvolvimento Científico (FADEC).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obreli-Neto, P.R., Nobili, A., de Oliveira Baldoni, A. et al. Adverse drug reactions caused by drug–drug interactions in elderly outpatients: a prospective cohort study. Eur J Clin Pharmacol 68, 1667–1676 (2012). https://doi.org/10.1007/s00228-012-1309-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1309-3