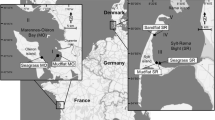
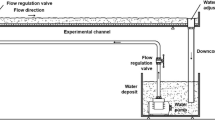
Abstract
Spatiotemporal distribution patterns in relation to feeding behavior of herbivorous gastropods have been studied extensively, but still knowledge about small-scale patterns is limited in relation to eutrophication. This experimental study aimed to describe the small-scale distribution of Littorina littorea in nutrient-enriched and nutrient-unenriched mesocosms in a merely atidal region and relate the distribution to food abundance and possible competing organisms, while checking simultaneously for feeding activities. The latter part was accomplished through the “gut fluorescence technique” GFT (which, to our knowledge, has not previously been used for benthic grazers) to estimate per capita grazing rates and the former part through monitoring of spatial heterogeneity of L. littorea and co-variation with sessile organisms (using semivariograms and cross-semivariograms, respectively). After 5 months of nutrient addition, the abundance and biomass of L. littorea had increased in enriched systems, which also had significantly higher total biomass of green algae. Gut pigment content was higher in L. littorea from enriched mesocosms, and gut depletion rate was higher in L. littorea from unenriched mesocosms. Spatial analysis showed that L. littorea exhibited generally random patterns (suggesting feeding activities) but sometimes (often in the morning) spatial patchiness (clumped distribution) in both enriched and unenriched conditions. There was mainly positive co-variation between L. littorea and biofilm, while different nutrient conditions exhibited contrasting co-variation between L. littorea and barnacles (positive co-variation in enriched and negative co-variation in unenriched mesocosms). The study offered insights into how feeding behavior and spatial distribution of a species may interact with community components differently under different nutrient regimes. The applied methodology can be useful for purposes of faster examination of grazing effects among different regions and also to compare grazing intensities and interactions between grazers and the benthic communities in disturbed (including pollution and nutrient enrichment) and non-disturbed systems, as well as in up-welling versus non-upwelling areas.







Similar content being viewed by others
References
Asmus H (1987) Secondary production of an intertidal mussel bed community related to its storage and turnover compartments. Mar Ecol Prog Ser 39:251–266
Båmstedt U, Gifford DJ, Irigoien X, Atkinson A, Roman A (2000) Feeding. In: Harris R, Wiebe P, Lenz J, Skjoldal HR (eds) ICES zooplankton methodology manual. Academic Press, London, pp 297–399
Bernard KS, Froneman PW (2005) Mesozooplankton Community structure and grazing impact in the Polar Frontal Zone of the South Indian Ocean during Austral Autumn 2002. Polar Biol 26:268–275
Bertness MD, Yund PO, Brown AF (1983) Snail grazing and the abundance of algal crusts on a sheltered New England rocky beach. J Exp Mar Biol Ecol 71:147–164
Boaventura D, Alexander M, Della Santina P, Smith ND, Re P, da Fonseca LC, Hawkins SJ (2002) The effects of grazing on the distribution and composition of low-shore algal communities on the central coast of Portugal and on the southern coast of Britain. J Exp Mar Biol Ecol 267:185–206
Bokn T, Lein TE (1978) Long-term changes in fucoid associations of inner Oslofjord, Norway. Norw J Bot 25:9–14
Bokn TL, Moy FE, Christie H, Engelbert S, Karez R, Kersting K, Kraufvelin P, Lindblad C, Marbà N, Pedersen MF, Sørensen K (2002) Are rocky shore ecosystems affected by nutrient enriched seawater? Some preliminary results from a mesocosm experiment. Hydrobiologia 484:167–175
Bokn TL, Duarte CM, Pedersen MF, Marbà N, Moy FE, Barrón C, Bjerkeng B, Borum J, Christie H, Engelbert S, Fotel FL, Hoell EE, Karez R, Kersting K, Kraufvelin P, Lindblad C, Olsen M, Sommer U, Sørensen K (2003) The response of experimental rocky shore communities to nutrient additions. Ecosystems 6:577–594
Boyd CM, Smith SL, Cowles TJ (1980) Grazing patterns of copepods in the upwelling systems off Peru. Limnol Oceanogr 25:583–596
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3128–3139
Buschbaum C (2000) Direct and indirect effects of Littorina littorea (L.) on barnacles growing on mussel beds in the Wadden Sea. Hydrobiologia 440:119–128
Carlson RL, Shulman MJ, Ellis JC (2006) Factors contributing to spatial heterogeneity in the abundance of the common periwinkle Littorina littorea. J Mol Stud 72:149–156
Chang AL, Blakeslee AMH, Miller AW, Ruiz GM (2011) Establishment failure in biological invasions: a case history of Littorina littorea in California, USA. PLOS One 6(1):e16035
Chapman MG (2000) Variability of foraging in highshore habitats: dealing with unpredictability. Hydrobiologia 426:75–87
Chapman MG, Underwood AJ (1996) Influence of tidal conditions, temperature and desiccation on patterns of aggregation of the high-shore periwinkle, Littorina unifasciata, in the New South Wales, Australia. J Exp Mar Biol Ecol 196:213–237
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Coleman RA (2010) Limpet aggregation does not alter desiccation in the limpet Cellana tramoserica. J Exp Mar Biol Ecol 386:113–118
Coleman RA, Benedetti-Cecchi L, Åberg P, Arenas F, Arrontes J, Castro J, Hartnoll RG, Jenkins SR, Paula J, Della Santina P, Underwood AJ, Hawkins SJ (2006) A continental scale evaluation of limpet grazing on rocky shores. Oecologia 147:556–564
Connell JH (1985) Variation and persistence of rocky shore populations. In: Moore PG, Seed R (eds) The ecology of rocky coasts. Hodder and Stoughton Educational Press, Kent, England, pp 57–69
Connell SD, Irwing AD (2009) The subtidal ecology of rocky coasts: local-regional biogeographic patterns and their experimental analysis. In: Witman JD, Kaustuv R (eds) Marine macroecology. University of Chicago Press, Chicago, pp 392–417
Dale MRT (2000) Spatial pattern analysis in plant ecology. Cambridge University Press, Cambridge. 326 pp
Diaz ER, McQuiad CD (2011) A spatially explicit approach to trophic interactions and landscape formation: patchiness in small-scale variability of grazing effects along an intertidal stress gradient. J Ecol 99:416–430
Diaz ER, Erlandsson J, McQuaid CD (2011) Detecting spatial heterogeneity in intertidal algal functional groups, grazers and their co-variation among shore levels and sites. J Exp Mar Biol Ecol 409:123–135
Durbin EG, Campbell RG (2007) Reassessment of the gut pigment method for estimating in situ zooplankton ingestion. Mar Ecol Prog Ser 331:305–307
Eriksson BK, Rubach A, Hillebrand H (2006) Biotic habitat complexity controls species diversity and nutrient effects on net biomass production. Ecology 87:246–254
Eriksson BK, Ljunggren L, Sandström A, Johansson G, Mattila J, Rubach A, Råberg S, Snickars M (2009) Declines in predatory fish promote bloom-forming macroalgae. Ecol Appl 19:1975–1988
Erlandsson J, McQuaid CD (2004) Spatial structure of recruitment in the mussel Perna perna at local scales: effects of adults, algae and recruit size. Mar Ecol Prog Ser 267:173–185
Erlandsson J, McQuaid CD, Kostylev V (2005) Contrasting spatial heterogeneity of sessile organisms within mussel (Perna perna L.) beds in relation to topographic variability. J Exp Mar Biol Ecol 314:79–97
Gamfeldt L, Hillebrand H, Jonsson P (2005) Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol Lett 8:696–703
Gray J, Naylor E (1995) Foraging and homing behaviour of the limpet Patella vulgata: a geographical comparison. J Moll Stud 62:121–124
Griffin J, Noël LM-LJ, Crowe TP, Burrows MT, Hawkins SJ, Thompson RC, Jenkins SR (2010) Consumer effects on ecosystem functioning in rock pools: roles of species richness and composition. Mar Ecol Prog Ser 420:45–56
Hawkins SJ, Hartnoll RG (1983) Grazing of intertidal algae by marine invertebrates. Oceanogr Mar Biol Annu Rev 21:195–282
Hillebrand H (2003) Opposing effects of grazing and nutrients on diversity. Oikos 100:592–600
Hillebrand H, Worm B, Lotze HK (2000) Marine microbenthic community structure regulated by nitrogen loading and grazing pressure. Mar Ecol Prog Ser 204:27–38
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
Hutchinson N, Williams GA (2003) An assessment of variation in molluscan grazing pressure on Hong Kong rocky shores. Mar Biol 142:495–507
Jaschinski S, Sommer U (2011) How do nutrient conditions and species identity influence the impact of mesograzers in eelgrass-epiphyte systems? Mar Biol 158:193–203
Johnson MP, Burrows MT, Hartnoll RG, Hawkins SH (1997) Spatial structure on moderately exposed rocky shores: patch scales and the interactions between limpets and algae. Mar Ecol Prog Ser 160:209–215
Jones KMM, Boulding EG (1999) State-dependent habitat selection by an intertidal snail: the costs of selecting a physically stressful microhabitat. J Exp Mar Biol Ecol 242:149–177
Karez R, Engelbert S, Kraufvelin P, Pedersen MF, Sommer U (2004) Biomass response and changes in composition of ephemeral macroalgal assemblages along an experimental gradient of nutrient enrichment. Aquat Bot 78:103–117
Kostylev V, Erlandsson J (2001) A fractal approach for detecting spatial hierarchy and structure on mussel beds. Mar Biol 139:497–506
Kostylev V, Erlandsson J, Johannesson K (1997) Microdistribution of the polymorphic snail Littorina saxatilis (Olivi) in a patchy rocky shore habitat. Ophelia 47:1–12
Kraufvelin P (1999) Baltic hard bottom mesocosms unplugged: replicability, repeatability and ecological realism examined by multivariate techniques. J Exp Mar Biol Ecol 240:229–258
Kraufvelin P (2007) Responses to nutrient enrichment, wave action and disturbance in rocky shore communities. Aquat Bot 87:262–274
Kraufvelin P, Christie H, Olsen M (2002) Littoral macrofauna (secondary) responses to experimental nutrient addition to rocky shore mesocosms and a coastal lagoon. Hydrobiologia 484:149–166
Kraufvelin P, Salovius S, Christie H, Moy FE, Karez R, Pedersen MF (2006a) Eutrophication-induced changes in benthic algae affect the behaviour and fitness of the marine amphipod Gammarus locusta. Aquat Bot 84:199–209
Kraufvelin P, Moy FE, Christie H, Bokn TL (2006b) Nutrient addition to experimental rocky shore communities revisited: delayed responses, rapid recovery. Ecosystems 9:1076–1093
Kraufvelin P, Lindholm A, Pedersen MF, Kirkerud LA, Bonsdorff E (2010) Biomass, diversity and production of rocky shore macroalgae at two nutrient enrichment and wave action levels. Mar Biol 157:29–47
Kristiansen S, Paasche E (1982) Nitrogen nutrition of the phytoplankton in the Oslofjord. Estuar Coast Shelf Sci 14:237–249
Little C (1989) Factors governing patterns of foraging activity in littoral marine herbivorous mollusks. J Moll Stud 55:273–284
Little C, Partridge J, Teagle L (1991) Foraging activity of limpets in normal and abnormal tidal regimes. J Mar Biol Assoc UK 71:537–554
Lubchenco J, Gaines SD (1981) A unified approach to marine plant-herbivore interactions. I. Populations and communities. Annu Rev Ecol Syst 12:405–437
Mackas D, Bohrer R (1976) Fluorescence analysis of zooplankton gut contents and an investigation of diel feeding patterns. J Exp Mar Biol Ecol 25:77–85
Madikiza LO (2005) The role of grazers and basal substrate cover in the control of intertidal algal distribution. MSc-thesis, University of Western Cape, South Africa
Masterson P, Arenas FA, Thompson RC, Jenkins SR (2008) Interaction of top down and bottom up factors in intertidal rockpools: effects on early successional macroalgal community composition, abundance and productivity. J Exp Mar Biol Ecol 363:12–20
McQuaid CD (1996) Biology of the gastropod family Littorinidae. II. Role in the ecology of intertidal and shallow marine ecosystems. Oceanogr Mar Biol Ann Rev 34:263–302
Newell RC et al (1971) Factors affecting feeding rate of winkle Littorina littorea. Mar Biol 9:138–144
Norton TA, Hawkins SJ, Manley NL, Williams GA, Watson DC (1990) Scraping a living: a review of littorinid grazing. Hydrobiologia 193:117–138
Paine RT (2002) Trophic control of production in a rocky intertidal community. Science 296:736–739
Pakhomov EA, Froneman PW (2004) Zooplankton dynamics in the eastern Atlantic sector of the Southern Ocean during the austral summer 1997/1998—Part 2: Grazing impact. Deep-Sea Res Pt II 51:2617–2631
Penry DL, Jumars PA (1986) Chemical reactor analysis and optimal digestion theory. Bioscience 36:310–315
Perez KA (1995) Role and significance of scale to ecotoxicology. In: Cairns J, Niederlehner BR (eds) Ecological toxicity testing: scale, complexity and relevance. Lewis Publishers, CRC Press, Boca Raton, pp 49–72
Perez KO, Carlson RL, Shulman MJ, Ellis JC (2009) Why are intertidal snails rare in the subtidal? Predation, growth and the vertical distribution of Littorina littorea (L.) in the Gulf of Maine. J Exp Mar Biol Ecol 369:79–86
Perissinotto R (1992) Mesozooplankton size-selectivity and grazing impact on phytoplankton community of the prince Edward Archipelago (Southern Ocean). Mar Ecol Prog Ser 79:243–258
Raffaelli DG, Hughes RN (1978) The effects of crevice size and availability on the population structure of Littorina rudis Maton. J Anim Ecol 47:71–83
Russell BD, Connell SD (2007) Responses of grazers to sudden nutrient pulses in oligotrophic versus eutrophic conditions. Mar Ecol Prog Ser 349:73–80
Scheiner SM, Gurevitch J (1993) Design and analysis of ecological experiments. Chapman and Hall, New York
Schmid PE (2000) Fractal properties of habitat and patch structure in benthic ecosystems. Adv Ecol Res 30:339–401
Skov MW, Volkelt-Igoe M, Hawkins SJ, Jesus B, Thompson RC, Doncaster CP (2010) Past and present grazing boosts the photo-autotrophic biomass of biofilms. Mar Ecol Prog Ser 401:101–111
Sokal RR, Rohlf FJ (1995) Biometry, the principles and practice of statistics in biological research, 3rd edn. WH Freeman and Company, New York, 887 pp
Taghon GL (1981) Beyond selection: optimal ingestion rate as a function of food value. Am Nat 118:202–214
Thompson RC, Crowe TP, Hawkins SJ (2002) Rocky intertidal communities, past environmental changes, present status and predictions for the next 25 years. Environ Conserv 29:168–191
Underwood AJ (1980) The effect of grazing by gastropods and physical factors on the upper limits of distribution of intertidal macroalgae. Oecologia 46:201–213
Underwood AJ, Chapman MG (1996) Scales of spatial patterns of distribution of intertidal invertebrates. Oecologia 107:212–224
Underwood AJ, Jernakoff P (1984) The effects of tidal height, wave exposure, seasonality and rock-pools on grazing and the distribution of intertidal macroalgae in the South Wales. J Exp Mar Biol Ecol 75:71–96
Valdivia N, Thiel M (2006) Effects of point-source nutrient addition and mussel removal on epibiotic assemblages in Perumytilus purpuratus beds. J Sea Res 56:271–283
Wahl M, Sönnichsen H (1992) Marine epibiosis. IV. The periwinkle Littorina littorea lacks typical antifouling defences–why are some populations so little fouled? Mar Ecol Prog Ser 88:225–235
Wahl M, Link H, Alexandridis N, Thomason J, Cifuentes M, Costello MJ, da Gama BAP, Hillock K, Hobday AJ, Kaufmann MJ, Keller S, Kraufvelin P, Krüger I, Lauterbach L, Antunes BL, Molis M, Nakaoka M, Nyström J, bin Radzi Z, Stockhausen B, Thiel M, Vance T, Weseloh A, Whittle M, Wiesmann L, Wunderer L, Yamakita T, Lenz M (2011) Re-structuring of marine communities exposed to environmental change: a global study on the interactive effects of species and functional richness. PLOS One 6(5):e19514
Wilhelmsen U, Reise K (1994) Grazing on green algae by the periwinkle Littorina littorea in the Wadden Sea. Helgolander Meeresun 48:233–242
Worm B, Lotze HK (2006) Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnol Oceanogr 51:569–579
Worm B, Lotze HK, Hillebrand H, Sommer U (2002) Consumer versus resource control of species diversity and ecosystem functioning. Nature 417:848–851
Acknowledgments
ED and JE were funded by Formas (The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning), whereas PK was funded by Svenska Kulturfonden. We are grateful to Hartvig Christie and Sofie Knutar for field assistance, to Per-Ivar Johannessen and Oddbjørn Pettersen for excellent daily maintenance of the Solbergstrand mesocosms, and to William Froneman and Kim Bernard for insights about the use of GFT. Hartvig Christie’s and Frithjof Moy’s scientific project SACCHARINA (from the Research Council of Norway 2007–2010) covered the costs for operative mesocosms during 2008 and their kind approval of our simultaneous research activities in the systems is highly appreciated. The Solbergstrand mesocosms can be viewed live at the web-cam link: http://151.157.160.150/view/index.shtml (username and password = guest).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Díaz, E.R., Kraufvelin, P. & Erlandsson, J. Combining gut fluorescence technique and spatial analysis to determine Littorina littorea grazing dynamics in nutrient-enriched and nutrient-unenriched littoral mesocosms. Mar Biol 159, 837–852 (2012). https://doi.org/10.1007/s00227-011-1860-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1860-y