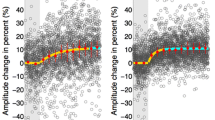
Abstract
In order to further our understanding of action-blindsight, four hemianopic patients suffering from visual field loss contralateral to a unilateral occipital lesion were compared to six healthy controls during a double task of verbally reported target detection and saccadic responses toward the target. Three oculomotor tasks were used: a fixation task (i.e., without saccade) and two saccade tasks (eliciting reflexive and voluntary saccades, using step and overlap 600 ms paradigms, respectively), in separate sessions. The visual target was briefly presented at two different eccentricities (5° and 8°), in the right or left visual hemifield. Blank trials were interleaved with target trials, and signal detection theory was applied. Despite their hemifield defect, hemianopic patients retained the ability to direct a saccade toward their contralesional hemifield, whereas verbal detection reports were at chance level. However, saccade parameters (latency and amplitude) were altered by the defect. Saccades to the contralesional hemifield exhibited longer latencies and shorter amplitudes compared to those of the healthy group, whereas only the latencies of reflexive saccades to the ipsilesional hemifield were altered. Furthermore, healthy participants showed the expected latency difference between reflexive and voluntary saccades, with the latter longer than the former. This difference was not found in three out of four patients in either hemifield. Our results show action-blindsight for saccades, but also show that unilateral occipital lesions have effects on saccade generation in both visual hemifields.






Similar content being viewed by others
Notes
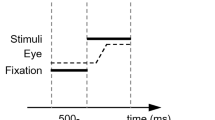
In the overlap procedure, the fixation point remains visible after the target’s appearance, either throughout the trial (cf. Leigh and Zee 2006, p110) or for a fixed duration of overlap (cf. Becker 1991, p114), the go signal being the target onset or the fixation point offset, respectively. The question of whether overlap saccades belong to the category of voluntary saccades is a matter of debate. Studies on saccadic adaptation have provided arguments that saccades elicited with an overlap procedure (durations above 400 ms) can be classified as voluntary, since adaptive changes in step saccades have not been found to transfer to overlap or scanning saccades (Deubel 1995, 1999 see also Collins and Doré-Mazars 2006; Alahyane et al. 2007), whereas adaptation has been found to transfer between overlap and scanning saccades. In order to compare reflexive and voluntary saccades with the most similar possible protocols, we compared saccades elicited by step and 600 ms overlap paradigms.
References
Alahyane N, Salemme R, Urquizar C, Cotti J, Guillaume A, Vercher JL, Pelisson D (2007) Oculomotor plasticity: are mechanisms of adaptation for reactive and voluntary saccades separate? Brain Res 1135:107–121
Azouvi P, Samuel C, Louis-Dreyfus A et al (2002) Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosur Psychiatry 73:160–166
Azzopardi P, Cowey A (1997) Is blindsight like normal, near-threshold vision? Proc Natl Acad Sci USA 94:14190–14194
Azzopardi P, Cowey A (1998) Blindsight and visual awareness. Conscious Cogn 7:292–311
Barbur J, Forsyth P, Findlay J (1988) Human saccadic eye movements in the absence of the geniculocalcarine projection. Brain 111:63–82
Barton J, Sharpe J (1997) Smooth pursuit and saccades to moving targets in blind hemifields. A comparison of medial occipital, lateral occipital and optic radiation lesions. Brain 120:681–699
Becker W (1989) Metrics. In: Wurtz RH, Goldberg ME (eds) The neurobiology of saccadic eye movements. Elseiver, New York, pp 13–67
Becker W (1991) Saccades. In: Carpenter R (ed) Vision and visual dysfunction, vol 8: eye movements. MacMillan, London
Blythe M, Kennard C, Ruddock K (1987) Residual vision in patients with retrogeniculate lesions of the visual pathways? Brain 110:887–905
Bodis-Wollner I, Bucher SF, Seelos KC, Paulus W, Reiser M, Oertel WH (1997) Functional MRI mapping of occipital and frontal cortical activity during voluntary and imagined saccades. Neurology 49:415–420
Campion J, Latto R, Smith Y (1983) Is blindsight an effect of scattered light, spared cortex, and near-threshold vision ? Behav Brain Sci 6:423–448
Cavézian C, Gaudry I, Perez C, Coubard O, Doucet G, Peyrin C, Marendaz C, Obadia M, Gout O, Chokron S (2010) Specific impairments in visual processing following lesion side in hemianopic patients. Cortex 46:1123–1131
Chokron S, Perez C, Obadia M, Gaudry I, Laloum L, Gout O (2008) From blindsight to sight: cognitive rehabilitation of visual field defects. Restor Neurol Neurosci 26:305–320
Collins T, Doré-Mazars K (2006) Eye movement signals influence perception: evidence from the adaptation of reactive and volitional saccades. Vis Res 46:3659–3673
Cowey A (2010) The blindsight saga. Exp Brain Res 200:3–24
Cowey A, Stoerig P, Bannister M (1994) Retinal ganglion cells labeled from the pulvinar nucleus in macaque monkeys. Neuroscience 61(3):691–705
Crawford J, Howell D (1998) Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol 12:482–486
Danckert J, Goodale M (2000) A conscious route to unconscious vision. Curr Biol 10:R64–R67
Danckert J, Rossetti Y (2005) Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev 29:1035–1046
Deubel H (1995) Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vis Res 35:3529–3540
Deubel H (1999) Separate adaptive mechanism for the control of reactive, volitional and memory-guided saccadic eye movements. In: Gopher D, Koriat A (eds) Attention Performance. MIT Press, Cambridge
Ferraina S, Paré M, Wurtz R (2002) Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol 87:845–858
Findlay JM, Walker R (1999) A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci 22:661–721
Frost D, Pöppel E (1976) Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: indications of a functional subdivision of the visual field. Biol Cybern 23:39–48
Gassel M, Williams D (1963) Visual function in patients with homonymous hemianopia. Part II. Oculomotor mechanisms. Brain 86:1–36
Gerardin P, Miquée A, Urquizar C, Pélisson D (2012) Functional activation of the cerebral cortex related to sensorimotor adaptation of reactive and voluntary saccades. Neuroimage 61:1100–1112
Goebel R, Muckli L, Zanella F, Singer W, Stoerig P (2001) Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res 41:1459–1474
Goodale M (1973) Cortico-tectal and intertectal modulation of visual responses in the rat’s superior colliculus. Exp Brain Res 17:75–86
Goodale M (1991) A neurological dissociation between perceiving objects and grasping them. Nature 349:154–156
Green DM, Swets JA (1966) Signal detection theory and psychophysics. Wiley, New York
Herter T, Guitton D (2004) Accurate bidirectional saccade control by a single hemicortex. Brain 127:1393–1402
Herter T, Guitton D (2007) Saccades to the seeing visual hemifield in hemidecorticate patients exhibit task-dependent reaction times and hypométrie. Exp Brain Res 182:11–25
Holmes G (1918) Disturbances of vision by cerebral lesions. Br J Ophthalmol 2:353–384
Holtzman J (1984) Interactions between cortical and subcortical visual areas: evidence from human commissurotomy patients. Vision Res 24:801–813
Ishiai S, Furukawa T, Tsukagoshi H (1987) Eye-fixation patterns in homonymous hemianopia and unilateral spatial neglect. Neuropsychologia 25:675–679
King S, Azzopardi P, Cowey A, Oxbury J, Oxbury S (1996) The role of light scatter in the residual visual sensitivity of patients with complete cerebral hemispherectomy. Vis Neurosci 13:1–13
Lalli S, Hussain Z, Ayub A, Cracco R, Bodis-Wollner I, Amassian V (2006) Role of the calcarine cortex (V1) in perception of visual cues for saccades. Clin Neurophysiol 117:2030–2039
Leigh R, Zee D (1980) Eye movements of the blind. Invest Ophthalmol Vis Sci 19:328–331
Leigh R, Zee B (2006) The neurology of eye movements. University Press, Oxford
Mc Millan NA, Creelman CD (1991) Detection theory: a user’s guide. Cambridge University Press, Cambridge
McDowell J, Dyckman K, Austin B, Clementz B (2008) Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn 68:255–270
Meienberg O, Zangemeister W, Rosenberg M, Hoyt W, Stark S (1981) Saccadic eye movement strategies in patients with homonymous hemianopia. Anna Neurol 9:537–544
Meienberg O, Harrer M, Wehren C (1986) Oculographic diagnosis of hemineglect in patients with homonymous hemianopia. J Neurol 233:97–101
Mort D, Perry R, Mannan S, Hodgson T, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C (2003) Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18:231–246
Perenin M, Jeannerod M (1975) Residual vision in cortically blind hemiphields. Neuropsychologia 13:1–7
Perenin MT, Jeannerod M (1978) Visual function within the hemianopic field following early cerebral hemidecortication in man: I. Spatial localization. Neuropsychologia 16:1–13
Perenin M, Rossetti Y (1996) Grasping without form discrimination in a hemianopic field. NeuroReport 7:793–797
Petit L, Zago L, Vigneau M, Andersson F, Crivello F, Mazoyer B, Mellet E, Tzourio-Mazoyer N (2009) Functional asymmetries revealed in visually guided saccades: an fMRI study. J Neurophysiol 102:2994–3003
Pierrot-Deseilligny C, Rivaud S, Penet C, Rigolet M (1987) Latencies of visually guided saccades in unilateral hemispheric cerebral lesions. Ann Neurol 21:138–148
Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch A (1995) Cortical control of saccades. Ann Neurol 37:557–567
Pierrot-Deseilligny C, Milea D, Müri R (2004) Eye movement control by the cerebral cortex. Curr Opin Neurol 17:17–25
Pöppel E, Richards W (1974) Light sensitivity in cortical scotomata contralateral to small islands of blindness. Exp Brain Res 21:125–130
Pöppel E, Held R, Frost D (1973) Residual visual function after brain wounds involving the central pathways in man. Nature 243:295–296
Richards W (1973) Visual processing in scotomata. Exp Brain Res 17:333–347
Rivaud S, Müri R, Gaymard B, Vermersch A, Pierrot-Deseilligny C (1994) Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res 102:110–120
Rizzo M, Robin D (1996) Bilateral effects of unilateral visual cortex lesions in human. Brain 119:951–963
Ross L, Ross S (1980) Saccade latency and warning signals: stimulus onset, offset, and change as warning events. Percept Psychophys 27:251–257
Rossetti Y (1998) Implicit short-lived motor representation of space in brain-damaged and healthy subjects. Conscious Cogn 7:520–558
Savina O, Bergeron A, Guitton D (2013) Blindsight after hemidecortication: visual stimuli in blind hemifield influence anti-saccades directed there. Cortex 49:861–876
Schärli H, Harman A, Hogben J (1999) Blindsight in subjects with homonymous visual field defects. J Cogn Neurosci 11:52–66
Schiller P, Tehovnik E (2001) Look and see: how the brain moves your eyes about. Prog Brain Res 134:127–142
Sharpe J, Lo A, Rabinovitch H (1979) Control of the saccadic and smooth pursuit systems after cerebral hemidecortication. Brain 102:387–403
Singer W, Zihl J, Pöppel E (1977) Subcortical control of visual thresholds in humans: evidence for modality specific and retinotopically organized mechanisms of selective attention. Exp Brain Res 29:173–190
Sommer M, Wurtz R (2004) What the brain tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91:1381–1402
Sprague J (1966) Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153:1544–1547
Stanislaw H, Todorov N (1999) Calculation of signal detection theory measures. Behav Res Methods Instrum Comput 31:137–149
Stoerig P, Hübner M, Pöppel E (1985) Signal detection analysis of residual vision in a field defect due to a post-geniculate lesion. Neuropsychologia 23:589–599
Trevethan C, Sahraie A, Weiskrantz L (2007) Can blindsight be superior to “sighted-sight”? Cognition 103:491–501
Valencia-Alfonso C, Verhaal J, Güntürkün O (2009) Ascending and descending mechanisms of visual lateralization in pigeons. Philos Trans R Soc Lond B Biol Sci 364:955–963
Walker R, Mannan S, Maurer D, Pambakian A, Kennard C (2000) The oculomotor distractor effect in normal and hemianopic vision. Proc Biol Sci 267:431–438
Weiskrantz L (1978) Some aspects of visual capacity in monkeys and man following striate cortex lesions. Arch Ital Biol 116:318–323
Weiskrantz L (1996) Blindsight revisited. Curr Biol 6:215–220
Weiskrantz L (2004) Roots of blindsight. Prog Brain Res 144:229–241
Weiskrantz L, Warrington E, Sanders M, Marschall J (1974) Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 97:709–728
Wurtz R (2009) Superior colliculus. In. LR Squire (eds) Encyclopedia of neuroscience. Academia Press, Oxford, pp 627–634
Wurtz R, Sommer M, Paré M, Ferraina S (2001) Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res 41(25–26):3399–3412
Yoshida M, Takaura K, Kato R, Ikeda T, Isa T (2008) Striate cortical lesions affect deliberate decision and control of saccade: implication for blindsight. J Neurosci 28:10517–10530
Zangemeister W, Meienberg O, Stark L, Hoyt W (1982) Eye-head coordination in homonymous hemianopia. J Neurol 226:243–254
Zih J (1999) Oculomotor scanning performance in subjects with homonymous visual field disorders. Vis Impair Res 1:23–31
Zihl J (1980) “Blindsight”: improvement of visually guided eye movements by systematic practice in patients with cerebral blindness. Neuropsychologia 18:71–77
Zihl J, Von Cramon D (1980) Registration of light stimuli in the cortically blind hemifield and its effect on localization. Behav Brain Res 1:287–298
Zihl J, Werth R (1984) Contributions to the study of “blindsight” –I. Can stray light account for saccadic localization in patients with postgeniculate field defects? Neuropsychologia 22:1–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fayel, A., Chokron, S., Cavézian, C. et al. Characteristics of contralesional and ipsilesional saccades in hemianopic patients. Exp Brain Res 232, 903–917 (2014). https://doi.org/10.1007/s00221-013-3803-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3803-y