Abstract
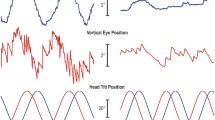
Head movements in a rotating frame of reference are commonly encountered, but their long term effects on the angular vestibulo-ocular reflex (aVOR) are not well understood. To study this, monkeys were oscillated about a naso-occipital (roll) axis for several hours while rotating about a spatial vertical axis (roll-while-rotating, RWR). This induced oscillations in roll and pitch eye velocity and continuous horizontal (yaw) nystagmus. For several hours thereafter, simple roll in darkness induced horizontal nystagmus and pitch and roll oscillations. The rising and falling time constants of the horizontal velocity indicated that the nystagmus arose in velocity storage. The continuous nystagmus was correlated with a phase shift of vertical eye velocity from 90° to 0° re head position. As the phases reverted toward pre-adaptive values, the horizontal velocity declined. Similar yaw nystagmus and pitch and roll velocities were produced by oscillation in roll after adaptation with roll and horizontal optokinetic nystagmus (OKN), but not after adaptation with pitch-while-rotating (PWR). Findings were explained by a model that shifted the roll orientation vector of velocity storage toward the pitch axis during adaptation with RWR and Roll & OKN. This shift produced modulation in vertical eye velocity in the post adaptive state, which was approximately in phase with roll head position, generating horizontal nystagmus. Similar orientation changes to prolonged exposure to complex motion environments may be responsible for producing post-stimulus motion sickness and/or mal de debarquement.







Similar content being viewed by others
References
Angelaki DE, Hess BJM (1998) Visually induced adaptation in three-dimensional organization of primate vestibuloocular reflex. J Neurophysiol 79:791–807
Baker J, Harrison RE, Isu N, Wickland C, Peterson B (1986) Dynamics of adaptive change in vestibulo-ocular reflex direction. II. Sagittal plane rotations. Brain Res 371:166–170
Bos JE, Bles W, de Graaf B (2002) Eye movements to yaw, pitch, and roll about vertical and horizontal axes: adaptation and motion sickness. Aviat Space Environ Med 73:436 434
Brown JJ, Baloh RW (1987) Persistent mal de debarquement syndrome: a motion-induced subjective disorder of balance. Am J Otolaryngol 8:219–222
Cha YH, Brodsky J, Ishiyama G, Sabatti C, Baloh RW (2008) Clinical features and associated syndromes of mal de debarquement. J Neurol 255:1038–1044
Cohen B, Raphan T (2004) The physiology of the vestibuloocular reflex. In: Highstein SM, Fay RR (eds) The vestibular system. Springer, Berlin, pp 235–285
Cohen B, Uemura T, Takemori S (1973) Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Equil Res 3:88–93
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol (Lond) 270:321–344
Cohen B, Dai M, Raphan T (2003) The critical role of velocity storage in production of motion sickness. Ann NY Acad Sci 1004:359–376
Cohen B, Dai M, Yakushin SB, Raphan T (2008) Baclofen, motion sickness susceptibility and the neural basis for velocity storage. Prog Brain Res 171:543–553
Crawford JD, Vilis T (1991) Axes of eye rotation and Listing’s law during rotations of the head. J Neurophysiol 65:407–423
Dai M, Raphan T, Cohen B (1991) Spatial orientation of the vestibular system: dependence of optokinetic after nystagmus on gravity. J Neurophysiol 66:1422–1438
Dai M, McGarvie L, Kozlovskaya IB, Raphan T, Cohen B (1994) Effects of spaceflight on ocular counterrolling and spatial orientation of the vestibular system. Exp Brain Res 102:45–56
Dai M, Klein A, Cohen B, Raphan T (1999) Model-based study of the human cupular time constant. J Vest Res 9:293–301
Dai M, Kunin M, Raphan T, Cohen B (2003) The relation of motion sickness to the spatial-temporal properties of velocity storage. Exp Brain Res 151:173–189
Dai M, Raphan T, Cohen B (2006) Effects of baclofen on the angular vestibulo-ocular reflex. Exp Brain Res 171:262–271
Darwin E (1796) Zoonomia. J. Johnson, London
de Wit G (1953) Seasickness (motion sickness). A labyrinthological study. Acta Otolaryngol Suppl 118:1–56
DiZio P, Lackner JR (2000) Congenitally blind individuals rapidly adapt to coriolis force perturbations of their reaching movements. J Neurophysiol 84:2175–2180
DiZio P, Lackner JR (2001) Coriolis-force-induced trajectory and endpoint deviations in the reaching movements of labyrinthine-defective subjects. J Neurophysiol 85:784–789
Eron JN, Cohen B, Raphan T, Yakushin SB (2008) Adaptation of orientation vectors of otolith-related central vestibular neurons to gravity. J Neurophysiol 100:1686–1690
Fernández C, Goldberg JM (1976) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force response relations. J Neurophysiol 39:985–995
Gizzi M, Rudolph S, Cohen B, Raphan T (1992) The representation of the spatial vertical in human optokinetic nystagmus. Ann NY Acad Sci 656:843–846
Goldberg JM, Fernandez C (1971a) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to angular accelerations. J Neurophysiol 34:635–660
Goldberg JM, Fernandez C (1971b) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. III. Variations among units in their discharge properties. J Neurophysiol 34:676–684
Gordon CR, Shupak A, Nachum Z (2000) Mal de debarquement. Arch Otolaryngol Head Neck Surg 126:805–806
Guedry FE Jr, Graybiel A (1962) Compensatory nystagmus conditioned during adaptation to living in a rotating room. J Appl Physiol 17:398–404
Hain TC, Hanna PA, Rheinberger MA (1999) Mal de debarquement. Arch Otolaryngol Head Neck Surg 125:615–620
Harrison RE, Baker JF, Isu N, Wickland CR, Peterson BW (1986) Dynamics of adaptive change in vestibulo-ocular reflex direction. I. Rotations in the horizontal plane. Brain Res 371:162–165
Hess BJ, Angelaki DE (1993) Angular velocity detection by head movements orthogonal to the plane of rotation. Exp Brain Res 95:77–83
Irwin J (1881) The pathology of sea-sickness. Lancet 2:907–909
Lackner JR, DiZio PA (2003) Adaptation to rotating artificial gravity environments. J Vestib Res 13:321–330
Lewis RF (2004) Frequency-specific mal de debarquement. Neurology 63:1983–1984
Moore ST, Clement G, Dai M, Raphan T, Cohen B (2003) Ocular and perceptual responses to linear acceleration in microgravity: alterations in otolith function on the COSMOS and Neurolab flights. J Vest Res 13:377–393
Nachum Z, Shupak A, Letichevsky V, Ben-David J, Tal D, Tamir A, Talmon Y, Gordon CR, Luntz M (2004) Mal de debarquement and posture: reduced reliance on vestibular and visual cues. Laryngoscope 114:581–586
Nooij SA, Bos JE, Groen EL (2008) Velocity storage activity is affected after sustained centrifugation: a relationship with spatial disorientation. Exp Brain Res 190(2):165–177
O’Hanlon JF, McCauley ME (1974) Motion sickness incidence as a function of the frequency of acceleration of vertical sinusoidal motion. Aerosp Med 45:366–396
Pettorossi VE, Errico P, Ferraresi A, Barmack NH (1999) Optokinetic and vestibular stimulation determines the spatial orientation of negative optokinetic afternystagmus in the rabbit. J Neurosci 19:1524–1531
Raphan T (2009) Velocity storage. In: Encyclopedia of neuroscience. Springer, Berlin (in press)
Raphan T, Cohen B (2002) The vestibulo-ocular reflex (VOR) in three dimensions. Exp Brain Res 145:1–27
Raphan T, Sturm D (1991) Modelling the spatiotemporal organization of velocity storage in the vestibuloocular reflex by optokinetic studies. J Neurophysiol 66:1410–1420
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35:229–248
Raphan T, Cohen B, Henn V (1983) Nystagmus generated by sinusoidal pitch while rotating. Brain Res 276:165–172
Raphan T, Dai MJ, Maruta J, Waespe W, Henn V, Suzuki J-I, Cohen B (1999) Canal and otolith afferent activity underlying eye velocity responses to pitching while rotating. Ann NY Acad Sci 871:181–194
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng BME 10:137–145
Shultheis L, Robinson D (1981) Directional plasticity of the vestibilo-ocular reflex in the cat. Ann NY Acad Sci 374:504–512
Skavenski AA, Robinson DA (1973) Role of abducens neurons in vestibuloocular reflex. J Neurophysiol 36:724–738
Waespe W, Henn V (1978) Reciprocal changes in primary and secondary optokinetic after-nystagmus (OKAN) produced by repetitive optokinetic stimulation in the monkey. Arch Psychiatr Nervenkr 225:23–30
Waespe W, Huber T, Henn V (1978) Dynamic changes of optokinetic after-nystagmus (OKAN) caused by brief visual fixation periods in monkey and in man. Arch Psychiatr Nervenkr 226:1–10
Wearne S, Raphan T, Cohen B (1998) Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol 79:2690–2715
Wertheim AH, Bos JE, Bles W (1998) Contributions of roll and pitch to sea sickness. Brain Res Bull 47:517–524
Yakushin SB, Dai MJ, Suzuki J-I, Raphan T, Cohen B (1995) Semicircular canal contribution to the three-dimensional vestibulo-ocular reflex: A model-based approach. J Neurophysiol 74:2722–2738
Yakushin SB, Raphan T, Cohen B (2000) Context-specific adaptation of the vertical vestibuloocular reflex with regard to gravity. J Neurophysiol 84:3067–3071
Yakushin SB, Ogorodnokov DA, Kunin M, Cohen B, Raphan T (2008) Dynamics of binocular fixation of targets during fore-aft motion. Prog Brain Res 171:303–311
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by DC007847, EY04148, DC05204, EY01867, DC05222.
Appendix
Appendix
In this appendix, we review the canal and otolith afferent activation patterns during pure pitch and roll as well as during PWR and RWR obtained from previous studies (Raphan et al. 1999). We also review the orientation vector organization of velocity storage (Dai et al. 1991; Raphan and Sturm 1991; Raphan and Cohen 2002; Cohen and Raphan 2004; Raphan 2009).
Canal and otolith activation during pure pitch, PWR, pure roll, and RWR
Studies of pitch and roll while rotating have shown that intact vertical semicircular canals are critical for activating velocity storage to generate the continuous horizontal nystagmus during both pitch while rotating and roll while rotating (Raphan et al. 1983). This work suggested that there was a correlation between the phases of the oscillatory vertical canal and otolith activity related to head position that generated the steady state continuous horizontal eye velocity response (Raphan et al. 1983; Raphan et al. 1999). Consistent with this prediction, activity of vertical canal afferents was close to being in phase with otolith afferent activity during pitch and roll while rotating (Raphan et al. 1999). How the head position and afferent vertical canal modulation frequencies are related in the various conditions of this study is shown diagrammatically in Fig. 7A. During pure pitch, the anterior canal activity oscillates at 90° phase leading head position. The posterior canal activity is 180° out of phase with the anterior canal activity (Fig. 8Aa). During PWR, both anterior and posterior canal activations oscillate closer in phase with head position as the planes of the vertical canals become maximally activated when they move closer to the plane of the constant spatial yaw rotation (Fig. 8Ab). During pure roll (Fig. 8Ac), anterior and posterior canal activities are in phase and lead Left Side Down postion (LSD) by 90°. During RWR (Fig. 8Ad), anterior canal activity is in phase with Right Side Down Head Position (RSD) as it is maximally activated when the plane of the canal is close to the plane of the rotation. The posterior canal phase also shifts, but is 180° out of phase with the anterior canal activation.
A Simulated modulation of semicircular canal activity during pure head pitch (a), PWR (b), pure head roll (c), and RWR (d). The simulations show Head Position (black trace), which is representative of otolith activation, as well as the modulation of the anterior and posterior canals for the left labyrinth. B The system matrix (H0) and its orientation vectors (eigenvectors) when the head is upright (a–c), tilted about a roll axis (d–f) and tilted about a pitch axis (g–i). When upright (a), the system matrix is diagonal (b), and the orientation vectors are orthogonal along the roll, pitch, and yaw axes of the head (c). When the head is tilted about a roll axis (d), the system matrix has a yaw to pitch cross-coupling term (hpy). The yaw eigenvector that stays along the spatial vertical. Similarly, when the head is tilted about a pitch axis (g), the system matrix has a yaw to pitch cross-coupling term (hry) and the yaw eigenvector stays along the spatial vertical. The pitch and roll orientation vectors remain along the head coordinate frame regardless of head tilt
The post-adaptive data suggest that the Canal-Otolith Correlator, shown in the model of Fig. 7a), does not receive information directly from the vertical canals and otolith afferents. Rather, it utilizes activity from the pitch component of velocity storage, which introduces a 90° phase shift relative to otolith afferent activity rather than directly from the vertical canal afferents (Fig. 7a, x). The output of the correlator then drives the horizontal mode of velocity storage (Fig. 7a). The postulate that the correlator utilizes input from the vertical mode of velocity storage would also explain why horizontal velocity was generated during Roll, following adaptation to Roll & OKN.
The lateral canal afferent activated oscillated at twice the frequency of oscillation of the vertical canal and otolith activity. Because of the lack of correlation between the lateral canals and the other signals, they could not have contributed to the continuous horizontal nystagmus during PWR or RWR. They contributed a double frequency oscillation in the horizontal nystagmus (Raphan et al. 1999).
Orientation vectors of velocity storage
Velocity storage has been modeled as a three-dimensional integrator, which has a system matrtix, H0, and an output represented by its state, x (Fig. 7a) (Dai et al. 1991; Raphan and Sturm 1991; Raphan and Cohen 2002; Cohen and Raphan 2004; Raphan 2009). Velocity storage is characterized by orientation vectors in a head coordinate frame that are represented by the eigenvectors of the system matrix (Dai et al. 1991; Raphan and Sturm 1991). Before adaptation, the system matrix H0, is diagonal (Fig. 8Bb), corresponding to orientation vectors (eigenvectors) of velocity storage that are approximately orthogonal in the upright position and are aligned with cardinal axes of the head (Fig. 8Bb, c). When the head is tilted about a head roll axis (Fig. 8Bd), the system matrix is no longer diagonal, but now has a cross coupling term from yaw to pitch, hpy and a yaw orientation that is aligned close to the spatial vertical (Fig. 8Be, f). Similarly, tilts about the pitch axis (Fig. 8Bg) introduce a cross coupling term, hry and a yaw orientation vector that is close to the spatial vertical. These kinds of static tilts have the effect of orienting the yaw orientation vector to the spatial vertical (Fig. 8Bf, i), creating a non-orthogonal basis for velocity storage. A consequence of this, is that yaw stimulation generates pitch or roll eye velocity so that the vector of eye velocity tends to align with the yaw orientation vector, which is no longer along the head yaw (Dai et al. 1991; Raphan and Sturm 1991). The pitch and roll orientation vectors move with the head and are linked to the body coordinate frame. The present study considers how a simple adaptation of the roll orientation vector could explain the data on post-adaptation with RWR.
Rights and permissions
About this article
Cite this article
Dai, M., Raphan, T. & Cohen, B. Adaptation of the angular vestibulo-ocular reflex to head movements in rotating frames of reference. Exp Brain Res 195, 553–567 (2009). https://doi.org/10.1007/s00221-009-1825-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1825-2