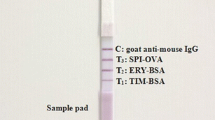
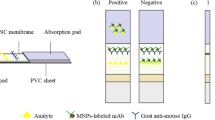
Abstract
A rapid, reliable, sensitive, and quantitative multi-residue fluorescent microspheres immunochromatographic assay (FMCA) was developed for simultaneous detection of four macrolides in raw milk. The IC50 value of the optimized FMCA was 1.36, 1.22, 1.01, and 1.39 ng/mL for erythromycin (ERY), spiramycin (SPI), tilmicosin (TIM), and tylosin (TYL), respectively. The limits of detection (LODs) for the four macrolides was 0.13 ng/mL. The recoveries of ERY, SPI, TIM, and TYL from spiked raw milk ranged from 91.8–109.2, 89.6–114.4, 84.8–111.6, and 85.8–115.2 %, respectively, with coefficients of variation (CVs) of 5.4–11.3, 7.9–15.7, 6.2–13.7, and 3.2–14.9 %, respectively. The whole testing process was completed within 20 min. The antibody-mixed labeled method was successfully applied to the FMCA, which greatly simplified the operation steps and saved a lot of time. Compared with the immunogold chromatographic assay (IGCA), the FMCA is more sensitive and stable and has less antibody consumption. A parallel analysis in blind raw milk samples was conducted by liquid chromatography–tandem mass spectrometry (LC-MS/MS); the results showed good correlation (r 2 = 0.99) between the two methods. Therefore, the developed multi-residue FMCA is reliable and can be easily applied to other antibiotics or other contaminants.

Multi-residue FMCA for simultaneous determination of macrolides in raw milk







Similar content being viewed by others
References
Glad C, Grubb AO (1981) Immuno-capillary-migration with enzyme-labeled antibodies—rapid quantification of c-reactive protein in human-plasma. Anal Biochem 116(2):335–340
Li X, Luo P, Tang S, Beier RC, Wu X, Yang L, Li Y, Xiao X (2011) Development of an immunochromatographic strip test for rapid detection of melamine in raw milk, milk products and animal feed. J Agric Food Chem 59(11):6064–6070
Byzova NA, Smirnova NI, Zherdev AV, Eremin SA, Shanin IA, Lei H-T, Sun Y, Dzantiev BB (2014) Rapid immunochromatographic assay for ofloxacin in animal original foodstuffs using native antisera labeled by colloidal gold. Talanta 119:125–132
Lin LR, Tong ML, Fu ZG, Dan B, Zheng WH, Zhang CG, Yang TC, Zhang ZY (2011) Evaluation of a colloidal gold immunochromatography assay in the detection of Treponema pallidum specific IgM antibody in syphilis serofast reaction patients: a serologic marker for the relapse and infection of syphilis. Diagn Microbiol Infect Dis 70(1):10–16
Hou W, Wang S, Wang X, Han X, Fan H, Cao S, Yue J, Wang Q, Jiang W, Ding C, Yu S (2015) Development of colloidal gold immunochromatographic strips for detection of Riemerella anatipestifer. PLoS ONE 10(3), e0122952
Meng K, Sun W, Zhao P, Zhang L, Cai D, Cheng Z, Guo H, Liu J, Yang D, Wang S, Chai T (2014) Development of colloidal gold-based immunochromatographic assay for rapid detection of Mycoplasma suis in porcine plasma. Biosens Bioelectron 55:396–399
Pan D, Pramanik M, Wickline SA, Wang LV, Lanza GM (2011) Recent advances in colloidal gold nanobeacons for molecular photoacoustic imaging. Contrast Media Mol Imaging 6(5):378–388
Liu Y, Wu A, Hu J, Lin M, Wen M, Zhang X, Xu C, Hu X, Zhong J, Jiao L, Xie Y, Zhang C, Yu X, Liang Y, Liu X (2015) Detection of 3-phenoxybenzoic acid in river water with a colloidal gold-based lateral flow immunoassay. Anal Biochem 483:7–11
Daohong Z, Peiwu L, Qi Z, Wen Z (2011) Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts. Biosens Bioelectron 26(6):2877–2882
Fan Z, Mingqiang Z, Yan C, Jinfeng L, Yanfei W, Xiaohua Q, Qiang X (2014) Lanthanide-labeled immunochromatographic strips for the rapid detection of Pantoea stewartii subsp. stewartii. Biosens Bioelectron 51:29–35
Taranova NA, Berlina AN, Zherdev AV, Dzantiev BB (2015) ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens Bioelectron 63:255–261
Liu D, Huang Y, Chen M, Wang S, Liu K, Lai W (2015) Rapid detection method for aflatoxin B-1 in soybean sauce based on fluorescent microspheres probe. Food Control 50:659–662
Xie QY, Wu YH, Xiong QR, Xu HY, Xiong YH, Liu K, Jin Y, Lai WH (2014) Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens Bioelectron 54:262–265
Xu D, Wu X, Li B, Li P, Ming X, Chen T, Wei H, Xu F (2013) Rapid detection of Campylobacter jejuni using fluorescent microspheres as label for immunochromatographic strip test. Food Sci Biotechnol 22(2):585–591
Wang Z, Li H, Li C, Yu Q, Shen J, De Saeger S (2014) Development and application of a quantitative fluorescence-based immunochromatographic assay for fumonisin B-1 in maize. J Agric Food Chem 62(27):6294–6298
Zhou J, Zhu K, Xu F, Wang W, Jiang H, Wang Z, Ding S (2014) Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine. J Agric Food Chem 62(49):12061–12066
Chen R, Li H, Zhang H, Zhang S, Shi W, Shen J, Wang Z (2013) Development of a lateral flow fluorescent microsphere immunoassay for the determination of sulfamethazine in milk. Anal Bioanal Chem 405(21):6783–6789
Pan FG, Fang Z, Meng RZ, Qin Y, Ju W, Liu J, Wu H (2013) Improving the sensitivity of fluorescent microsphere immunoassays for the simultaneous detection of foodborne pathogens using dimethylacetamide. Anal Lett 46(14):2203–2212
Li X, Wen K, Chen Y, Wu X, Pei X, Wang Q, Liu A, Shen J (2015) Multiplex immunogold chromatographic assay for simultaneous determination of macrolide antibiotics in raw milk. Food Anal Methods 8(9):2368–2375
Horie M, Saito K, Ishii R, Yoshida T, Haramaki Y, Nakazawa H (1998) Simultaneous determination of five macrolide antibiotics in meat by high-performance liquid chromatography. J Chromatogr A 812(1–2):295–302
Wang J, Leung D, Lenz SP (2006) Determination of five macrolide antibiotic residues in raw milk using liquid chromatography-electrospray ionization tandem mass spectrometry. J Agric Food Chem 54(8):2873–2880
Lee JM, Edwards HHL, Pereira CA, Samii SI (1996) Crosslinking of tissue-derived biomaterials in 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC). J Mater Sci Mater Med 7(9):531–541
Damink L, Dijkstra PJ, vanLuyn MJA, vanWachem PB, Nieuwenhuis P, Feijen J (1996) In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials 17(7):679–684
Peng DP, Ye SQ, Wang YL, Chen DM, Tao YF, Huang LL, Liu ZL, Dai MH, Wang XQ, Yuan ZH (2012) Development and validation of an indirect competitive enzyme-linked immunosorbent assay for the screening of tylosin and tilmicosin in muscle, liver, milk, honey and eggs. J Agric Food Chem 60(1):44–51
Albrecht U, Hammer P, Heeschen W (1996) Chicken antibody based ELISA for the detection of spiramycin in raw milk. Milchwissenschaft 51(4):209–212
Albrecht U, Walte HG, Hammer P (1998) Detection of erythromycin in raw milk by an antibody-capture-immunoassay. Kieler Milchwirtschaftliche Forschungsberichte 50(2):163–170
Jiang WX, Zhang HY, Li XM, Liu XX, Zhang SX, Shi WM, Shen JZ, Wang ZH (2013) Monoclonal antibody production and the development of an indirect competitive enzyme-linked immunosorbent assay for screening spiramycin in milk. J Agric Food Chem 61(46):10925–10931
Zhang JK, Qi YH, Liu JX, Wang JP (2013) Heterologous immunoassay for screening macrolide antibiotics residues in milk based on the monoclonal antibody of tylosin. Food Agric Immunol 24(4):419–431
Acknowledgments
This work was supported financially by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2015BAK36B03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, X., Shen, J., Wang, Q. et al. Multi-residue fluorescent microspheres immunochromatographic assay for simultaneous determination of macrolides in raw milk. Anal Bioanal Chem 407, 9125–9133 (2015). https://doi.org/10.1007/s00216-015-9078-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9078-3