Abstract
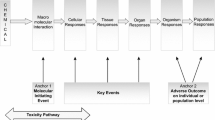
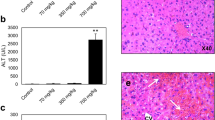
Adverse outcome pathways (AOPs) have been recently introduced as tools to map the mechanisms underlying toxic events relevant for chemical risk assessment. AOPs particularly depict the linkage between a molecular initiating event and an adverse outcome through a number of intermediate key events. An AOP has been previously introduced for cholestatic liver injury. The objective of this study was to test the robustness of this AOP for different types of cholestatic insult and the in vitro to in vivo extrapolation. For this purpose, in vitro samples from human hepatoma HepaRG cell cultures were exposed to cholestatic drugs (i.e. intrahepatic cholestasis), while in vivo samples were obtained from livers of cholestatic mice (i.e. extrahepatic cholestasis). The occurrence of cholestasis in vitro was confirmed through analysis of bile transporter functionality and bile acid analysis. Transcriptomic analysis revealed inflammation and oxidative stress as key events in both types of cholestatic liver injury. Major transcriptional differences between intrahepatic and extrahepatic cholestatic liver insults were observed at the level of cell death and metabolism. Novel key events identified by pathway analysis included endoplasmic reticulum stress in intrahepatic cholestasis, and autophagy and necroptosis in both intrahepatic as extrahepatic cholestasis. This study demonstrates that AOPs constitute dynamic tools that should be frequently updated with new input information.





Similar content being viewed by others
Abbreviations
- AOP:
-
Adverse outcome pathway
- ABC:
-
ATP-binding cassette family
- ATA:
-
Atazanavir
- ATF:
-
Activation transcription factor
- BA(s):
-
Bile acid(s)
- BDL:
-
Bile duct ligation
- BSEP:
-
Bile salt export pump
- CA:
-
Cholic acid
- CAR:
-
Constitutive androstane receptor
- CCR:
-
C–C chemokine receptor type
- CDCA:
-
Chenodeoxycholic acid
- CHOP:
-
CCAAT-enhancer-binding protein homologous protein
- CIx:
-
Cholestatic index
- CLF:
-
Cholyl-l-lysyl-fluorescein
- CsA:
-
Cyclosporin A
- CSF:
-
Colony stimulating factor
- CYLD:
-
Cylindromatosis
- CYP:
-
Cytochrome P450
- DILI:
-
Drug-induced liver injury
- DMSO:
-
Dimethyl sulfoxide
- DCA:
-
Deoxycholic acid
- Fos:
-
Fos proto-oncogene
- FXR:
-
Farnesoid X receptor
- GCA:
-
Glycocholic acid
- GCDCA:
-
Glycochenodeoxycholic acid
- GDCA:
-
Glycodeoxycholic acid
- Gst:
-
Glutathione S-transferase
- Il(1rl1):
-
Interleukin (1 receptor-like 1)
- IPA:
-
Ingenuity Pathway Analysis
- IRAK:
-
Interleukin 1 receptor-associated kinase
- JUN:
-
Jun proto-oncogene
- MAP1LC3β:
-
Microtubule-associated protein 1 light chain 3β
- MAPKAPK:
-
Mitogen-activated protein kinase-activated protein kinase
- MDR:
-
Multidrug resistance protein
- MLKL:
-
Mixed lineage kinase domain-like pseudokinase
- MRP:
-
Multidrug resistance-associated protein
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NEFA:
-
Nefazodone
- NR(s):
-
Nuclear receptor(s)
- NTCP:
-
Sodium-taurocholate co-transporting polypeptide
- Nqo:
-
NAD(P)H quinone dehydrogenase
- OATP(s):
-
Organic anion transporting peptide(s)
- OST:
-
Organic solute transporter
- PXR:
-
Pregnane X receptor
- RIPK:
-
Receptor interacting serine/threonine kinase
- SD:
-
Standard deviation
- SERPINE1:
-
Serpin E1
- SHP:
-
Small heterodimer partner
- SH3GLB1:
-
SH3 domain containing GRB2 like, endophilin B1
- SLC(O):
-
Solute carrier (organic anion transporter) family
- SQSTM:
-
Sequestosome
- UGT:
-
UDP-glucuronosyltransferase
References
Afonso MB, Rodrigues PM, Simão AL et al (2016) Activation of necroptosis in human and experimental cholestasis. Cell Death Dis 7:e2390
Ankley GT, Bennett RS, Erickson RJ et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741
Anthérieu S, Bachour-El Azzi P, Dumont J et al (2013) Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 57:1518–1529
Arduini A, Serviddio G, Tormos AM et al (2012) Mitochondrial dysfunction in cholestatic liver diseases. Front Biosci 4:2233–2252
Bachour-El Azzi P, Sharanek A, Burban A et al (2015) Comparative localization and functional activity of the main hepatobiliary transporters in HepaRG cells and primary human hepatocytes. Toxicol Sci 145:157–168
Bale SS, Vernetti L, Senutovitch N et al (2014) In vitro platforms for evaluating liver toxicity. Exp Biol Med 239:1180–1191
Begriche K, Massart J, Robin MA et al (2011) Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol 54:773–794
Bhat TA, Chaudhary AK, Kumar S et al (2017) Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim Biophis Acta 1867:58–66
Bissio E, Lopardo GD (2013) Incidence of hyperbilirubinemia and jaundice due to atazanavir in a cohort of hispanic patients. AIDS Res Hum Retroviruses 29(3):415–417
Botla R, Spivey JR, Aguilar H et al (1995) Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther 272:930–938
Burban A, Sharanek A, Guguen-Guillouzo C et al (2018) Endoplasmic reticulum stress precedes oxidative stress in antibiotic-induced cholestasis and cytotoxicity in human hepatocytes. Free Radic Biol Med 115:166–178
Burbank MG, Sharanek A, Burban A et al (2017) Mechanistic insights in cytotoxic and cholestatic potential of the endothelial receptor antagonists using HepaRG cells. Toxicol Sci 157:451–464
Chatterjee S, Richert L, Augustijns P et al (2014) Hepatocyte-based in vitro model for assessment of drug-induced cholestasis. Toxicol Appl Pharmacol 274:124–136
Copple BL, Jaeschke H, Klaassen CD (2010) Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis 30:195–204
Dawson S, Stahl S, Paul N et al (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40:130–138
Dewaele D, Annaert P, Hoeben E (2019) LC-MS/MS analysis of bile acids in in vitro samples. Methods Mol Biol 1981:15–23
Dragovic S, Vermeulen NPE, Gerets HH et al (2016) Evidence-based selection of training compounds for use in the mechanism-based integrated prediction of drug-induced liver injury in man. Arch Toxicol 90:2979–3003
Gao L, Lv G, Guo X et al (2014) Activation of autophagy protects against cholestasis-induced hepatic injury. Cell Biosci 4:47
Gijbels E, Vilas-Boas V, Deferm N et al (2019) Mechanisms and in vitro models of drug-induced cholestasis. Arch Toxicol 93:1169–1186
Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KBF, Reddy KR, Haynes K, Roy J, Sha D, Marks AR, Schneider JL, Strom BL, Corley DA, Lo Re V (2015) Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 148(7):1353–1361.e3
Gores GJ, Miyoshi H, Botla R et al (1998) Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis: a potential role for mitochondrial proteases. Biochim Biophys Acta 1366:167–175
Halilbasic E, Baghdasaryan A, Trauner M (2013) Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis 17:161–189
Hendriks DFG, Puigvert LF, Messner S et al (2016) Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability. Sci Rep 6:35434
Henkel AS, LeCuper B, Olivares S et al (2017) Endoplasmic reticulum stress regulated hepatic bile acid metabolism in mice. Cell Mol Gastroenterol Hepatol 3:261–271
Humbert L, Maubert MA, Wolf C et al (2012) Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B 899:135–145
Jones SC, Kortepeter C, Brinker AD (2018) Postmarketing surveillance of drug-induced liver injury. Drug-induced liver toxicity. Methods Pharmacol Toxicol. https://doi.org/10.1007/978-1-4939-7677-5_22
Kmiéc Z (2001) Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161:1–151
Kolarić TO, Ninčević V, Smolić R et al (2019) Mechanisms of hepatic cholestatic drug injury. J Clin Transl Hepatol 7:86–92
Kostrubsky SE, Strom SC, Kalgutkar AS et al (2006) Inhibition of hepatobiliary transport as a predictive method for clinical hepatotoxicity of nefazodone. Toxicol Sci 90:451–459
Laverty HG, Antoine DJ, Benson C et al (2010) The potential of cytokines as safety biomarkers for drug-induced liver injury. Eur J Clin Pharmacol 66:961–976
Lee WM (2013) Drug-induced acute liver failure. Clin Liver Dis 17:575–586
Lepist EI, Gillies H, Smith W et al (2014) Evaluation of the endothelin receptor antagonists ambrisentan, bosentan, macitentan, and sitaxsentan as hepatobiliary transporter inhibitors and substrates in sandwich- cultured human hepatocytes. PLoS ONE 9:e87548
Liu R, Li X, Huang Z et al (2018) C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice. Hepatology 67:1441–1457
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54:795–809
McGill MR, Yan HM, Ramachandran A et al (2011) HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53:974–982
Manley S, Ni HM, Kong B et al (2014) Suppression of autophagic flux by bile acids in hepatocytes. Toxicol Sci 137:478–490
Mariotti V, Strazzabosco M, Fabris L et al (2017) Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta 1864:1254–1261
Morgan RE, Trauner M, van Staden CJ et al (2010) Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118:485–500
Nguyen KD, Sundaram V, Ayoub WS (2014) Atypical causes of cholestasis. World J Gastroenterol 20:9418–9426
Noor F (2015) A shift in paradigm towards human biology-based systems for cholestatic-liver diseases. J Physiol 593:5043–5055
Oorts M, Baze A, Bachellier P et al (2016) Drug-induced cholestasis risk assessment in sandwich-cultured human hepatocytes. Toxicol In Vitro 34:179–196
Parent R, Marion MJ, Furio L et al (2004) Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology 126:1147–1156
Qu X, Zhang Y, Zhang S, Zhai J, Gao H, Tao L, Song Y (2018) Dysregulation of BSEP and MRP2 may play an important role in isoniazid-induced liver injury via the SIRT1/FXR pathway in rats and HepG2 cells. Biol Pharm Bull 41(8):1211–1218
Rakotondravelo S, Poinsignon Y, Borsa-Lebas F et al (2012) Complicated atazanavir-associated cholelithiasis: a report of 14 cases. Clin Infect Dis 55:1270–1272
Riede J, Poller B, Huwyler J et al (2017) Assessing the risk of drug-induced cholestasis using unbound intrahepatic concentrations. Drug Metab Dispos 45:523–531
Rodrigues RM, Kollipara L, Chaudhari U et al (2018) Omics-based responses induced by bosentan in human hepatoma HepaRG cell cultures. Arch Toxicol 92:1939–1952
Roman ID, Fernandez-Moreno MD, Fueyo JA et al (2003) Cyclosporin A induced internalization of the bile salt export pump in isolated rat hepatocyte couplets. Toxicol Sci 71:276–281
Schoemaker MH, Conde de la Rosa L, Buist-Homan M et al (2004) Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 39:1563–1573
Seok J, Warren HS, Cuenca AG et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110:3507–3512
Sharanek A, Bachour-El Azzi P, Al-Attrache H et al (2014) Different dose-dependent mechanisms are involved in early cyclosporine-A induced cholestatic effects in HepaRG cells. Toxicol Sci 141:244–253
Sharanek A, Burban A, Humbert L et al (2015) Cellular accumulation and toxic effects of bile acids in cyclosporine A-treated HepaRG hepatocytes. Toxicol Sci 147:573–587
Sharanek A, Burban A, Humbert L et al (2017) Progressive and preferential cellular accumulation of hydrophobic bile acids induced by cholestatic drugs is associated with inhibition of their amidation and sulfatation. Drug Metab Dispos 45:1292–1303
Tag CG, Sauer-Lehnen S, Weiskirchen S et al (2015) Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp 96:52438
Tagliacozzi D, Mozzi AF, Casetta B et al (2003) Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 41:1633–1641
Van Campenhout S, Van Vlierberghe H, Devisscher L (2019) Common bile duct ligation as model for secondary biliary cirrhosis. Methods Mol Biol 1981:237–247
Van den Hof WF, Ruiz-Aracama A, Van Summeren A et al (2015) Integrating multiple omics to unravel mechanisms of cyclosporin A induced hepatotoxicity in vitro. Toxicol In Vitro 29:489–501
Vatakuti S, Olinga P, Pennings JLA, Groothuis GMM (2017) Validation of precision-cut liver slices to study drug-induced cholestasis: a transcriptomics approach. Arch Toxicol 91(3):1401–1412
Villeneuve DL, Crump D, Garcia-Reyero N et al (2014) Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci 142:312–320
Vinken M, Landesmann B, Goumenou M et al (2013) Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol Sci 136:97–106
Woolbright BL, Jaeschke H (2012) Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol 18:4985–4993
Yasumiba S, Tazuma S, Ochi H et al (2001) Cyclosporin A reduces canalicular membrane fluidity and regulates transporter function in rats. Biochem J 354:591–596
Zhang D, Chango TJ, Everett DW et al (2005) In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos 33:1729–1739
Zhang J, Kan H, Cai L et al (2016) Inhibition of bile salt transport by drugs associated with liver injury in primary hepatocytes from human, monkey, dog, rat and mouse. Chem Biol Interact 255:45–54
Zollner G, Marschall HU, Wagner M et al (2006) Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm 3:231–251
Zollner G, Trauner M (2006) Molecular mechanisms of cholestasis. Wien Med Wochenschr 156:380–385
Acknowledgements
This work was supported by grants of the Research Foundation Flanders, Belgium and the Scientific Fund Willy Gepts, Belgium and the Center for Alternatives to Animal Testing at Johns Hopkins University, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gijbels, E., Vilas‐Boas, V., Annaert, P. et al. Robustness testing and optimization of an adverse outcome pathway on cholestatic liver injury. Arch Toxicol 94, 1151–1172 (2020). https://doi.org/10.1007/s00204-020-02691-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02691-9