Abstract
Summary
By analyzing iron status of 14 ADHR patients, we found that iron deficiency was an important trigger of ADHR. Correcting iron deficiency significantly improved patients’ symptoms. Meanwhile, patients’ serum phosphate showed positive correlations with iron metabolism parameters and hemoglobin-related parameters, suggesting the necessity of monitoring and correcting the iron status in ADHR.
Introduction
Autosomal dominant hypophosphatemic rickets (ADHR) is unique for its incomplete penetrance, variety of disease onsets, and waxing and waning phenotypes. Iron deficiency is a trigger of ADHR. This study aimed to clarify the role of iron deficiency in ADHR.
Methods
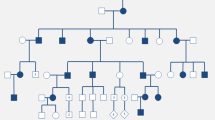
Data of clinical manifestations and laboratory examinations were collected from patients among eight kindreds with ADHR. Multiple regression and Pearson’s correlation tests were performed to test the relationships of serum phosphate levels and other laboratory variables during the patients’ follow-ups.
Results
Among 23 ADHR patients with fibroblast growth factor 23 (FGF23) mutations, 14 patients presented with obvious symptoms. Ten patients had iron deficiency at the onset of ADHR, coinciding with menarche, menorrhagia, pregnancy, and chronic gastrointestinal bleeding. Two patients who did not have their iron status tested presented with symptoms after abortion and pregnancy in one patient each, which suggested that they also had iron deficiency at onset. Patients were treated with ferrous succinate tablets, vitamin C, and neutral phosphate and calcitriol. With correction of the iron status, the patients’ symptoms showed notable improvement, with increased serum phosphate levels. Two patients’ FGF23 levels also declined to the normal range. There were strong correlations between serum phosphate and serum iron levels (r = 0.7689, p < 0.0001), serum ferritin levels (r = 0.5312, p = 0.002), iron saturation (r = 0.7907, p < 0.0001), and transferrin saturation (r = 0.7875, p < 0.001). We also examined the relationships between serum phosphate levels and hemoglobin-related indices, which were significant (hemoglobin: r = 0.71, p < 0.0001; MCV: r = 0.7589, p < 0.0001; MCH: r = 0.8218, p < 0.0001; and MCHC: r = 0.7751, p < 0.0001). Longitudinal data of six patients’ follow-up also showed synchronous changes in serum phosphate with serum iron levels.
Conclusions
Iron deficiency plays an important role in triggering ADHR. Monitoring and correcting the iron status are helpful for diagnosing and treating ADHR. Iron metabolism parameters and hemoglobin-related parameters are positively correlated with serum phosphate levels in patients with ADHR and iron deficiency, and these might serve as good indicators of prognosis of ADHR.



Similar content being viewed by others
References
Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82(2):674–681. https://doi.org/10.1210/jcem.82.2.3765
Imel EA, Hui SL, Econs MJ (2007) FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22(4):520–526. https://doi.org/10.1359/jbmr.070107
Consortium A (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26(3):345–348. https://doi.org/10.1038/81664
Gribaa M, Younes M, Bouyacoub Y, Korbaa W, Ben Charfeddine I, Touzi M, Adala L, Mamay O, Bergaoui N, Saad A (2010) An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J Bone Miner Metab 28(1):111–115. https://doi.org/10.1007/s00774-009-0111-5
Bai XY, Miao D, Goltzman D, Karaplis AC (2003) The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278(11):9843–9849. https://doi.org/10.1074/jbc.M210490200
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60(6):2079–2086. https://doi.org/10.1046/j.1523-1755.2001.00064.x
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19(3):429–435. https://doi.org/10.1359/JBMR.0301264
Kato K, Jeanneau C, Tarp MA et al (2006) Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 281(27):18370–18377. https://doi.org/10.1074/jbc.M602469200
Tagliabracci VS, Engel JL, Wiley SE et al (2014) Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A 111(15):5520–5525. https://doi.org/10.1073/pnas.1402218111
Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE (2014) Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res 29(2):361–369. https://doi.org/10.1002/jbmr.2049
Farrow EG, Yu X, Summers LJ et al (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A 108(46):E1146–E1155. https://doi.org/10.1073/pnas.1110905108
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ (2011) Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 96(11):3541–3549. https://doi.org/10.1210/jc.2011-1239
Liu C, Zhao Z, Wang O, Li M, Xing X, Hsieh E, Fukumoto S, Jiang Y, Xia W (2019) Earlier onset in autosomal dominant hypophosphatemic rickets of R179 than R176 mutations in fibroblast growth factor 23: report of 20 Chinese cases and review of the literature. Calcif Tissue Int 105(5):476–486. https://doi.org/10.1007/s00223-019-00597-y
Furuyama K, Kaneko K, Vargas PD (2007) Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J Exp Med 213(1):1–16. https://doi.org/10.1620/tjem.213.1
Gkouvatsos K, Papanikolaou G, Pantopoulos K (2012) Regulation of iron transport and the role of transferrin. Biochim Biophys Acta 1820(3):188–202. https://doi.org/10.1016/j.bbagen.2011.10.013
Kassebaum NJ, Jasrasaria R, Naghavi M et al (2014) A systematic analysis of global anemia burden from 1990 to 2010. Blood 123(5):615–624. https://doi.org/10.1182/blood-2013-06-508325
Hanudel MR, Chua K, Rappaport M et al (2016) Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am J Physiol Renal Physiol 311(6):F1369–F1377. https://doi.org/10.1152/ajprenal.00281.2016
Koury MJ, Haase VH (2015) Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol 11(7):394–410. https://doi.org/10.1038/nrneph.2015.82
Hanudel MR, Eisenga MF, Rappaport M et al (2019) Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant 34(12):2057–2065. https://doi.org/10.1093/ndt/gfy189
Toro L, Barrientos V, Leon P et al (2018) Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int 93(5):1131–1141. https://doi.org/10.1016/j.kint.2017.11.018
Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D (2014) FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem 289(14):9795–9810. https://doi.org/10.1074/jbc.M113.527150
Agoro R, Montagna A, Goetz R, Aligbe O, Singh G, Coe LM, Mohammadi M, Rivella S, Sitara D (2018) Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J 32(7):3752–3764. https://doi.org/10.1096/fj.201700667R
Nam KH, Kim H, An SY et al (2018) Circulating fibroblast growth factor-23 levels are associated with an increased risk of anemia development in patients with nondialysis chronic kidney disease. Sci Rep 8(1):7294. https://doi.org/10.1038/s41598-018-25439-z
Rossaint J, Unruh M, Zarbock A (2017) Fibroblast growth factor 23 actions in inflammation: a key factor in CKD outcomes. Nephrol Dial Transplant 32(9):1448–1453. https://doi.org/10.1093/ndt/gfw331
Ganz T, Nemeth E (2016) Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol 36(2):87–93. https://doi.org/10.1016/j.semnephrol.2016.02.001
Kapelari K, Kohle J, Kotzot D, Hogler W (2015) Iron supplementation associated with loss of phenotype in autosomal dominant hypophosphatemic rickets. J Clin Endocrinol Metab 100(9):3388–3392. https://doi.org/10.1210/jc.2015-2391
Imel EA, Liu Z, Coffman M, Acton D, Mehta R, Econs MJ (2020) Oral iron replacement normalizes fibroblast growth factor 23 in iron-deficient patients with autosomal dominant hypophosphatemic rickets. J Bone Miner Res 35(2):231–238. https://doi.org/10.1002/jbmr.3878
Menon LP, Weinstein RS (2020) Iron replacement ameliorates hypophosphatemia in autosomal dominant hypophosphatemic rickets: a review of the role of iron. Bone 131:115137. https://doi.org/10.1016/j.bone.2019.115137
Imel EA, Gray AK, Padgett LR, Econs MJ (2014) Iron and fibroblast growth factor 23 in X-linked hypophosphatemia. Bone. 60:87–92. https://doi.org/10.1016/j.bone.2013.12.001
Hogler W, Kapelari K (2020) Oral iron for prevention and treatment of rickets and osteomalacia in autosomal dominant hypophosphatemia. J Bone Miner Res 35(2):226–230. https://doi.org/10.1002/jbmr.3941
WHO (2011) Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. (WHO:NMH:NHD:MNM:11.2)
Acknowledgments
We appreciate our patients and their family for their participation in this study.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (Nos. 81970757 and 81670714), National Key Program of Clinical Science (WBYZ2011-873), and the CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-3-003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our study was approved by the Institutional Review Boards of Peking Union Medical College Hospital (PUMCH). Written informed consent was obtained from all subjects.
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Li, X., Zhao, Z. et al. Iron deficiency plays essential roles in the trigger, treatment, and prognosis of autosomal dominant hypophosphatemic rickets. Osteoporos Int 32, 737–745 (2021). https://doi.org/10.1007/s00198-020-05649-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05649-w