Abstract
Summary
Adherence to anti-osteoporosis medications is poor. We carried out a cohort study using a real-world population database to estimate the persistence of anti-osteoporosis drugs. Unadjusted 2-year persistence ranged from 10.3 to 45.4%. Denosumab users had a 40% lower risk of discontinuation at 2 years compared to alendronate users.
Purpose
The purpose of this study was to estimate real-world persistence amongst incident users of anti-osteoporosis medications.
Methods
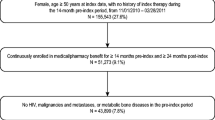
This is a retrospective cohort using data from anonymised records and dispensation data (www.sidiap.org). Eligibility comprised the following: women aged ≥50, incident users of anti-osteoporosis medication (2012), with data available for at least 12 months prior to therapy initiation. Exclusions are other bone diseases/treatments and uncommon anti-osteoporosis drugs (N < 100). Follow-up was from first pharmacy dispensation until cessation, end of study, censoring or switching. Outcomes are 2- and 1-year persistence with a permissible gap of up to 90 days. Persistence with alendronate was compared to other bisphosphonates, strontium ranelate, selective oestrogen receptor modulators, teriparatide and denosumab. Cox models were used to estimate hazard ratios of therapy cessation according to drug used after adjustment for age, sex, BMI, smoking, alcohol drinking, Charlson co-morbidity index, previous fractures, use of anti-osteoporosis medication/s, oral corticosteroids and socio-economic status.
Results
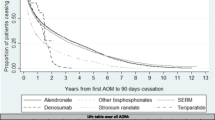
A total of 19,253 women were included. Unadjusted 2-year persistence [95% CI] ranged from 10.3% [9.1–11.6%] (strontium ranelate) to 45.4% [43.1–47.8%] (denosumab). One-year persistence went from 35.8% [33.9%–37.7%] (strontium ranelate) to 65.8% [63.6%–68.0%] (denosumab). At the end of the first year and compared to alendronate users, both teriparatide and denosumab users had reduced cessation risk (adjusted HR 0.76, 95% CI 0.67–0.86 and 0.54, 95% CI 0.50–0.59 respectively) while at the end of the second year, only denosumab had a lower risk of discontinuation (adjusted HR 0.60, 95% CI 0.56–0.64).
Conclusions
Unadjusted 2-year persistence is suboptimal. However, both teriparatide and denosumab users had better 1-year persistence and only denosumab had 2-year better persistence compared to alendronate users. Unmeasured confounding by indication might partially explain our findings.



Similar content being viewed by others
References
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Gallagher AM, Rietbrock S, Olson M, van Staa TP (2008) Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res 23:1569–1575
van Boven JF, de Boer PT, Postma MJ, Vegter S (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570
Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD (2012) Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 166:735–741
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P (2012) Persistence and compliance of medications used in the treatment of osteoporosis—analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 50:315–322
Netelenbos JC, Geusens PP, Ypma G, Buijs SJ (2011) Adherence and profile of non-persistence in patients treated for osteoporosis—a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 22:1537–1546
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23:223–231
Compston JE, Seeman E (2006) Compliance with osteoporosis therapy is the weakest link. Lancet 368:973–974
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Carbonell-Abella C, Pages-Castella A, Nogués X, Javiad MK, Arden NK, Cooper C, Diez-Perez A, Prieto-Alhambra D (2013) Persistence with different antiosteoporosis medications: A population-based cohort study. Osteoporos Int 24(Supp 1):S87–S384
Sicras Mainar A, Navarro Artieda R, Gutiérrez Nicuesa L, Sorio Vilela F, Intorcia M (2011) Continuance of treatment for osteoporosis in postmenopausal patients in the primary care setting. Farm Aten Primaria 9:46–53
Hadji P, Intorcia M, Thellmann K, Steinle T, Eisen C, Schimd T (2013) GRAND 3: the German retrospective cohort analysis on non-adherence in osteoporotic patients 3: persistence analysis of female patients treated with denosumab. Osteoporos Int 24(Suppl 1):S87–S384
Lakatos P, Tóth E, Cina Z, Lang Z, Psachoulia E, Intorcia M (2013) Persistence & compliance to treatment for osteoporosis in postmenopausal women in Hungary: a retrospective cohort study. Value Health 16:A567–A568
Karlsson L, Lundkvist J, Intorcia M, Psachoulia E, Ström O (2013) Treatment persistence in Swedish women initiating denosumab for treatment of postmenopausal osteoporosis. Value Health 16:A567
Hadji P, Papaioannou NA, Gielen E, Tepie MF, Zhang E, Kalouche-Khalid L, Fahrleitner-Pammer A (2014) 12-month persistence with Denosumab (Dmab) in women with postmenopausal osteoporosis (pmo): interim results of a 24-month prospective observational study in Germany, Austria, Greece and Belgium. Osteoporos Int 25(Suppl 2):S159–S440
Hadji P, Claus V, Kostev K, Intorcia M, Steinle T (2010) GRAND—the German retrospective cohort analysis on non-adherence in osteoporosis: analysis of persistence with intravenous bisphosphonates in women. Osteoporos Int 21(Suppl 1):S25–S388
Garcia-Gil Mdel M, Hermosilla E, Prieto-Alhambra D, Fina F, Rosell M, Ramos R, Rodriguez J, Williams T, Van Staa T, Bolibar B (2011) Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care 19:135–145
Borrell C, Marí-Dell’olmo M, Serral G, Martínez-Beneito M, Gotsens M, Members MEDEA (2010) Inequalities in mortality in small areas of eleven Spanish cities (the multicenter MEDEA project). Health Place 16:703–711
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB (2009) Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15:728–740
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
Arden NK, Earl S, Fisher DJ, Cooper C, Carruthers S, Goater M (2006) Persistence with teriparatide in patients with osteoporosis: the UK experience. Osteoporos Int 17:1626–1629
Ziller V, Zimmermann SP, Kalder M, Ziller M, Seker-Pektas B, Hellmeyer L, Hadji P (2010) Adherence and persistence in patients with severe osteoporosis treated with teriparatide. Curr Med Res Opin 26:675–681
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL, Investigators DAPS (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
DiMatteo MR, Haskard KB, Williams SL (2007) Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 45:521–528
Klop C, Welsing PM, Elders PJ, Overbeek JA, Souverein PC, Burden AM, van Onzenoort HA, Leufkens HG, Bijlsma JW, de Vries F (2015) Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int 26:1831–1840
Acknowledgements
We thank all the health professionals involved in registering data in computerised medical records for SIDIAP (Information System for Development of Primary Care Research). We thank as well Francisco Sorio Vilela and Laura Canals from Amgen for their contribution. Amgen provided comments on the design of the study protocol and the analysis plan. The final protocol and analysis plan were mutually agreed by SIDIAP and Amgen, based on the principle of the “best science known in the research field.” Amgen also provided comments on the publication prior to its submission to the journal. However, SIDIAP alone decided whether to incorporate Amgen comments in the submitted publication. Co-funded with FEDER funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Scientific approval was obtained from the SIDIAP Scientific Committee, and ethics approval was granted by the relevant board (CEIC IDIAP Jordi Gol). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
DPA’s research team has received unrestricted research funding from Bioiberica, Amgen and Servier Laboratoires; DPA’s department has received fees for speaker services from Amgen. C.T reports personal fees from Fundació Jordi Gol i Gurina, during the conduct of the study, and personal fees from Boehringer Ingelheim, outside the submitted work. DML reports fees for speaker or consultant services from Amgen, Lilly, MSD and Servier. CR, ASC, CCA and MSA report no conflict of interest.
Funding
Unrestricted research grant from Amgen. Partial funding of researchers (DPA) from NIHR Musculoskeletal Biomedical Research Unit Oxford, University of Oxford, UK.
Rights and permissions
About this article
Cite this article
Reyes, C., Tebe, C., Martinez-Laguna, D. et al. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28, 2997–3004 (2017). https://doi.org/10.1007/s00198-017-4144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4144-7