Abstract
Introduction
This narrative review focusing on critical care echocardiography (CCE) has been written by a group of experts in the field, with the aim of outlining the state of the art in CCE in the 10 years after its official recognition and definition.
Results
In the last 10 years, CCE has become an essential branch of critical care ultrasonography and has gained general acceptance. Its use, both as a diagnostic tool and for hemodynamic monitoring, has increased markedly, influencing contemporary cardiorespiratory management. Recent studies suggest that the use of CCE may have a positive impact on outcomes. CCE may be used in critically ill patients in many different clinical situations, both in their early evaluation of in the emergency department and during intensive care unit (ICU) admission and stay. CCE has also proven its utility in perioperative settings, as well as in the management of mechanical circulatory support. CCE may be performed with very simple diagnostic objectives. This application, referred to as basic CCE, does not require a high level of training. Advanced CCE, on the other hand, uses ultrasonography for full evaluation of cardiac function and hemodynamics, and requires extensive training, with formal certification now available. Indeed, recent years have seen the creation of worldwide certification in advanced CCE. While transthoracic CCE remains the most commonly used method, the transesophageal route has gained importance, particularly for intubated and ventilated patients.
Conclusion
CCE is now widely accepted by the critical care community as a valuable tool in the ICU and emergency department, and in perioperative settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Critical care echocardiography has become an essential branch of critical care ultrasonography and has gained general acceptance. Its use, both as a diagnostic tool and for hemodynamic monitoring, has increased markedly, influencing contemporary cardiorespiratory management. |
Introduction
The increasing availability of ultrasonography at the bedside has undeniably impacted greatly on critical care medicine practice. While impossible to quantify fully, this impact can be appreciated from the number of international professional bodies that now mandate competency in critical care ultrasonography (CCUS), as well as the formal certification processes now implemented internationally, and the proliferation of ultrasound-related academic publications. The fact that Cholley and colleagues’ 2006 appeal for greater use of critical care echocardiography [1] led, within 10 years, to the accumulation of sufficient academic work to allow the publication of international evidence-based guidelines [2], must certainly be seen as a clear indication of the rate of progress in this field.
Many clinicians will be familiar with the term “point-of-care ultrasonography” (POCUS), which typically means a goal-directed ultrasonography exam performed by the treating physician to answer a well-defined question relevant to the patient’s immediate care. More specific terminology and definitions were set out in 2009 as the result of a collaboration between the American College of Chest Physicians (ACCP) and the Société de Réanimation de Langue Française (SRLF) [3]. CCUS is the appropriate umbrella term for ultrasonography performed by intensivists, and its two main branches are critical care echocardiography (CCE) and general critical care ultrasonography (GCCUS). CCE can be divided into basic and advanced skill sets, and both basic and advanced CCE can be performed using either a transthoracic (TTE) or a transesophageal (TEE) approach, depending on the clinical questions at hand. Even though TEE is often regarded as a component of advanced CCE, and TTE as a more basic component, some clinicians may acquire competency first in TEE. The specific ultrasonography skills that comprise the basic and advanced CCE skill sets will be further described later in this article. The clinician performing a CCE exam is responsible for the acquisition and interpretation of the images, as well as the integration of these findings into the broader clinical picture. For this reason, he or she must have received thorough psychomotor and cognitive training, especially for the more advanced applications. There is also a need for humility: CCE providers must understand their own strengths and limitations, as well as those of the specific ultrasonography tools they propose to use.
This narrative review is part of a series of reviews on CCUS written for this journal. Focusing solely on CCE, it has been written by a group of recognized experts in the field, with the aim of outlining the state of the art in CCE 10 years after its official recognition and definition by the ACCP and SRLF [3]. CCE is here presented and discussed as a semi-continuous tool that can be used for the early evaluation of critically ill patients in the emergency department and following their admission to the intensive care unit (ICU). This continuity is also seen in the case of patients requiring surgery before or after ICU admission, as CCE may be used in the operating room as well. The article also provides some perspectives on the future, and discusses the key points and persisting uncertainties recognized by the experts (Fig. 1, Table 1).
CCE: increasing uptake but significant gaps
Although echocardiography is increasingly used in the critical care field, a review of the literature reveals that within this field there are numerous specific areas and clinical situations in which it is not used. A recently published paper based on the US National Inpatient Sample reported that the absolute volume of echocardiography increased at a rate of 3.4% a year between 2001 and 2011, even though it is very plausible that many of the studies were performed by cardiologists or sonographers rather than intensivists, and that the figures reported likely underestimate the rate of use of the technique in the critical care setting [4]. However, the same study reported that in critically ill septic patients, as well as those with congestive heart failure, echocardiography was used more than pulmonary artery catheterization (PAC), and also concluded that echo was still underutilized among patients who died during hospitalization [4]. Meanwhile, a French study found that the use of CCE in acute respiratory distress syndrome (ARDS) increased from 54.5 to 58.8% of patients between 2004–2006 and between 2010–2012, respectively, meaning that around 40% did not get the chance to benefit from the technique [5]. During the same period, the use of PAC decreased from 10.4 to 7.4% [5]. In a recent paper, experts discussed the value of CCE as a possible alternative to PAC [6], even though PAC may still currently be preferable for some indications, such as post-cardiac surgery ICU care. It was recently confirmed that there is a clinically acceptable correlation between systolic pulmonary artery pressure when measured by PAC or calculated by echocardiography, providing a direct measurement of central venous pressure is done [7]. In a 1-day prospective observational study performed in 140 European ICUs, Zieleskiewicz et al. reported that 1073 GCCUS exams were performed in 36% of the hospitalized patients, a large majority of these being basic CCE procedures for diagnosis, but also therapeutic adjustment [8]. In this study, TEE accounted for less than 10% of the studies performed [8], confirming that TTE is more commonly used due to its ease of use compared with TEE. In addition to its conventional role in cardiology diagnostics, CCE is now recognized as a hemodynamic monitoring tool, too [9]. However, in the FENICE study, echo variables were used in only 2% of the 2213 patients to guide fluid management, although in more than 40% of cases physicians did not use any variable [10]. It is clear that wide variations exist in the use of CCE. Accordingly, one major future goal should be to standardize CCE indications and use.
CCE: two different levels of competency
It is important to differentiate between basic and advanced CCE (Fig. 2). Basic CCE is understood as a five-view 2D TTE examination in which basic measurements, such as ventricular diameter, are obtained, with or without the use of the M-mode. Inspired by the early work of Jensen et al. [11], it was popularized under the acronym “FATE” (focused assessed TTE). Formalized structured learning programs such as focused echo in emergency life support and focused intensive care echo [12] are the legacy of this work. Basic CCE is designed to answer a simple binary question, such as whether the left or right ventricles are significantly impaired, or to identify the presence of a large pericardial effusion [13]. Although cardiology organizations were initially reluctant to recognize the value of CCE, they have now accepted the importance of its application [14]. Basic CCE is focused on rapidly categorizing and guiding the management of the patient with hemodynamic failure; there is no better alternative for initial and serial evaluation of the patient with shock. Advanced CCE allows the intensivist to deploy echocardiography with a similar capability as the cardiologist in order to more fully define the pathophysiology of cardiopulmonary failure. Advanced CCE utilizes the full range of two-dimensional views and Doppler-based measurements, and it requires a much higher level of cognitive and technical training than basic CCE [15].
CCE: two complementary imaging routes
The international consensus statement on training standards for advanced CCE stipulates that advanced CCE demands competency in both TTE and TEE [15]. While the two methods are complementary, they each have distinct advantages and disadvantages. A major advantage of TTE is that it is non-invasive and carries no risk to the patient. Furthermore, the setup time is minimal, and it can be quickly deployed at the point of care, and readily repeated as needed. The probe can be cleaned rapidly, allowing the operator to perform multiple scans in different patients in a short period of time. The probe design is compact, making it adaptable to the small, portable machines that are well designed for ICU work. TTE also provides better alignment than TEE for Doppler measurement of tricuspid regurgitation velocity, left ventricular outflow obstruction velocity, and transvalvular aortic flow velocity, and it is superior for two-dimensional imaging of superficial cardiac structures (apical thrombus, anterior pericardial space). However, a major shortcoming of TTE is possible inadequate image quality due to patient-specific factors (e.g. body habitus, presence of dressings, drains and devices, hyperinflation, inability to position the critically ill patient for optimal image acquisition). A greater than 10% weight gain compared with admission weight, a positive end-expiratory pressure ≥ 15 cm H2O, and chest tubes have been reported to be risk factors for failure of TTE imaging, which occurred in 38% of cases and was resolved by TEE in a study performed in trauma patients [16]. Compared with TEE, the training period required for TTE is longer and the technique is more operator dependent. For certain applications, TTE has limited capacity; these include evaluation for superior vena cava size/respirophasic variation, endocarditis, aortic dissection, localized pericardial hematoma with tamponade after cardiac surgery, left atrial thrombus, guidance of double-lumen extracorporeal membrane oxygenation (ECMO) catheter insertion/positioning, detailed analysis of native/prosthetic valve morphology, and diagnosis of acute cor pulmonale [17].
Conversely, as indicated, the training period required for TEE is shorter and the technique is less operator dependent, as it overcomes the patient-specific limitations of TTE. For certain applications, TEE is superior to TTE on account of its better image resolution (vide supra). Apart from its enhanced diagnostic capability in certain pathologies, it is a similarly effective tool for hemodynamic evaluation. In the critical care setting, TEE has certain limitations. While it has a lower complication rate compared with other typical critical care procedures such as endotracheal intubation and central venous access, it is not without risks. Esophageal injury, hypopharyngeal injury, and displacement of tracheostomy tube are rare or very rare complications [18]; most complications have been reported in spontaneously breathing awake patients [19], while critical care TEE is generally performed on patients who are receiving ventilatory support, unlike TEE performed by the cardiologist; so complication rates with TEE in the critically-ill may be lower than those reported in the field of cardiology. Cardiologists currently use echocardiography contrast agents rather than TEE to assess ventricular function in difficult patients; however, to date this is less used in critical care due to concerns relating to the high incidence of patients with acute severe respiratory disease. The setup time for TEE and the need for probe decontamination place time constraints on its rapid deployment and repeated use in the ICU. Another major limitation of TEE, unrelated to the technique itself, derives from the fact that many intensive care teams do not yet have a probe for their own, unrestricted use. This is more of a problem in North America, although it is also encountered in many European and Asian countries. This situation will change with the inevitable dissemination of this useful technology.
As already indicated, critical care TEE performed by intensivists is generally limited to patients who are on mechanical ventilatory support, while TTE is the standard procedure for non-intubated patients. Some skilled operators may choose to perform TEE in the non-intubated patient, if there is a clinical indication to do so. Given its ease of use, however, TTE should be the initial “go-to” technique unless there is a clearly defined indication that requires TEE imaging. In an intubated patient, where TTE imaging is inadequate to provide the answer, then provided there is no contraindication TEE should follow. In summary, TTE is an effective imaging modality for most patients in the intensive care unit but we recommend that TEE be available to the intensivist as a standard ICU tool.
Clinical applications and contexts
The role of echocardiography in the management of the critically ill is now well established (Table 2). From its incorporation into routine ICU practice for purposes ranging from rapid diagnosis to full hemodynamic evaluation and monitoring in circulatory shock, the use of CCE has gained momentum. However, it is crucial not to underestimate the challenges to be comprehended and the limitations and pitfalls to be overcome [20]. In this section we present some snapshots of a variety of contexts in which CCE is used and the various applications available. This is not an exhaustive account and the reader is invited to refer to the tables and figures for a broader overview.
CCE as a diagnostic tool
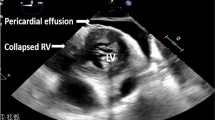
The diagnostic impact of CCE spans a wide range of pathologies commonly encountered in critical care (Fig. 3). Many providers view ultrasonography as an extension of the physical examination, and the integration of CCE with an admission history and physical examination makes for greater diagnostic accuracy [21,22,23,24,25]. In mechanically ventilated patients [26] and in those with unexplained hypotension [27], the addition of CCE improves the diagnostic yield and may alter the plan of care. Ample data now exist to suggest that clinicians can readily be trained to accurately perform basic CCE. Point-of-care assessments of left ventricular (LV) systolic function provide the most supportive evidence [28,29,30,31,32], as pericardial fluid collections which can reliably be ruled in or out by CCE [33,34,35]. Assessment of right ventricular (RV) function—this may include tricuspid annular plane systolic excursion and ventricular septal positioning—and valvular assessment have been less well studied, presumably as they are more complex.
Echocardiography is recommended for the evaluation of patients with symptoms consistent with a cardiac etiology (video 1 ESM) to aid in the diagnosis of myocardial infarction and for the evaluation of cardiac trauma [36]. Basic cardiac and lung ultrasonography can be used to accurately diagnose acute decompensated heart failure and the combination outperforms traditional tools such as physical examination, chest X-ray and laboratory studies [37,38,39]. Advanced CCE may be used to provide an evaluation of LV diastolic function, which no other hemodynamic monitoring tool can provide [40, 41]. There is increasing recognition that LV diastolic dysfunction is associated with higher mortality in patients with septic shock, and a greater incidence of failed weaning from ventilatory support. Hemodynamic monitoring has thus far focused almost exclusively on LV output, but RV assessment with CCE is important given the incidence of RV failure in patients with ARDS [42, 43].
Some of the earliest evidence in favor of CCE involved trauma patients, in whom its immediate use for evaluation of penetrating cardiac injury led to faster detection of pericardial effusions, faster time to operative management, and improved survival [44]. More recently the use of ultrasonography to assist in the resuscitation of a mixed population of trauma patients was shown to have an important impact in terms of improved detection and correction of hypovolemia and LV dysfunction, shorter triage times to surgery, and a trend towards lower mortality [45]. Identifying a reversible cause of arrest, such as a pericardial tamponade, can lead to a significant change in management and the potential to improve patient outcomes [46]. In patients presenting in the early phase of undifferentiated shock, routine application of CCE in the emergency department expedites the identification of the underlying etiology [47, 48] (video 2 ESM).
CCE as a hemodynamic monitoring tool
CCE has long been recognized as a valuable bedside tool for hemodynamic monitoring. A statement issued by 16 experts in the field recommended that CCE be used if patients were still in shock after initial fluid resuscitation, in order to evaluate cardiac function and rule out cardiac tamponade, and then be repeated as necessary to assess the impact of therapies on cardiac function [9]. CCE can be performed to measure cardiac output (CO). Rather than measuring an absolute value of CO, it has been reported that CCE is more reliable in tracking its changes [49], since converting the aortic velocity time index (VTI) into stroke volume require accurate measures of aortic diameter (which can give rise to errors), whereas changes in VTI can be measured reliably independently of aortic diameter. Thus, CCE can easily be used to evaluate the dynamic parameters needed for functional hemodynamic monitoring [50], i.e. for assessing fluid responsiveness. Stroke volume variation induced by positive-pressure breathing is estimated as VTI variation, and the dynamic changes in CO in response to passive leg raising (PLR) are assessed by comparing instantaneous and continuous VTI values before and during PLR. Conversely, the PAC-derived thermodilution technique for estimating CO averages CO values over minutes. However, CCE is much more than just a tool for measuring CO. By directly visualizing the different structures of the heart, it gives the intensivist specific and direct information on both LV and RV function, and offers many additional options for evaluating the need for fluid or pharmacological support [51]. Figure 4 summarizes the main echo parameters (measured or qualitatively evaluated) used for performing a global hemodynamic evaluation, together with their respective views and routes. From a hemodynamic monitoring perspective, the main difference between TTE and TEE is related to the evaluation of fluid responsiveness. While TTE evaluation of fluid responsiveness is based mainly on aortic VTI variations, CO changes in response to PLR, and measures of inferior vena cava (IVC) diameter (end-expiratory diameter and respiratory variations), the later with limited accuracy [52, 53], TEE also allows measures of superior (but not inferior) vena cava diameter changes with its respiratory variations (∆SVC). ∆SVC is reported to have very good specificity and moderate sensitivity for determination of volume responsiveness [52]; in all cases the dynamic parameters were validated in mechanically ventilated patients synchronized with the ventilator. CCE also provides an accurate evaluation of LV filling pressure when compared with PAC, allowing physicians to check for fluid tolerance. Thus, thanks to its ability to evaluate steady-state cardiac function and beat-to-beat changes relative to the respiratory cycle, CCE gives valuable information about heart–lung interactions in both mechanically and spontaneously ventilated patients (video 3) [54,55,56].
Main parameters for hemodynamic monitoring using critical care echocardiography, according to the route of echocardiography. a–c Longitudinal upper esophageal view at the SVC level with a complete collapse during insufflation (a, fluid responsiveness), no collapse (b, no fluid responsiveness), or intermediate respiratory variations (c, gray zone). d–f Subcostal view visualizing IVC, with virtual IVC and dilatation during insufflation (d, fluid responsiveness), normal size with dilatation during insufflation (e, fluid responsiveness), large IVC without any respiratory variation (f, no fluid responsiveness). g, h short axis view of the left ventricle by a transgastric approach. i parasternal short axis view of the left ventricle showing a paradoxical septal motion (arrow). j–l transverse mid-esophageal view with a normal right ventricle (j, RV/LV end-diastolic area ≤ 0.6), a moderate RV dilatation (k, RV/LV > 0.6) and a severe RV dilatation (l, RV > LV). TEE transesophageal echocardiography, TTE transthoracic echocardiography, ∆SVC respiratory variation of the superior vena cava, ∆IVC respiratory variation of the inferior vena cava, IVCEE end-expiratory inferior vena cava, LVFAC left ventricular fractional area contraction, LVEF left ventricular ejection fraction, WMA wall motion abnormality, RV right ventricle
A recent multicenter study in septic shock reported a moderate agreement between hemodynamic measurement performed using transpulmonary thermodilution (TPT) and CCE, and suggested a potential source of inaccuracy with TPT in 28% of cases [57]. Although discontinuous, serial CCE, when associated with continuous monitoring of invasive blood pressure, may be sufficient to manage most unstable patients [58]. A unique quality of CCE is that it allows individualization of hemodynamic management in addition to informing the ventilator settings and strategy. This is particularly relevant for evaluating RV function in the patient with ARDS [43, 59] or other processes associated with increased pulmonary arterial pressure. Development of new CCE technologies, like small-diameter TEE probes that can be left in place in patients for extended periods of time, should expand the utility of CCE in monitoring hemodynamics across wider spectra of patients [60,61,62]. TPT can also be used to estimate extravascular lung water. Although the utility of this value in isolation is unclear, B lines detected by lung ultrasonography report similar quantitative data, the more B lines the more lung water.
CCE for prognostication in septic shock
We have established that the management plan may be altered in as many as half of all cases as a result of CCE [63, 64], This may be based on a simple analysis of 2D imaging in septic shock [65]. CCE can aid in the management of septic patients by providing a diagnosis of septic cardiomyopathy (video 4 ESM), although there is limited support for the prognostic value of 2D LV ejection fraction (LVEF) conversely to ventriculo-arterial uncoupling [66]. More sophisticated assessment of LV function beyond visual assessment may require training in advanced CCE. Other than mitral annular planar systolic excursion, which is a simple M-mode measurement, speckle tracking echocardiography and global longitudinal strain (GLS) are advanced measurements which may allow earlier diagnosis of septic cardiomyopathy and assist in prognostication [67,68,69]. Sanfilippo et al. conducted a meta-analysis of eight studies, including 794 patients, that reported GLS and LVEF, and whilst LVEF did not show an association with mortality, worse GLS values (i.e. less negative) were associated with higher mortality in septic patients [70]. The large MIMIC-III database reported 6361 patients admitted to ICU with sepsis; in this population, early use of TTE had a significant benefit in terms of 28-day mortality, with more fluids administered during the first day and greater use of dobutamine [71]. Patients who had an echo also seemed to be more quickly weaned from vasopressors [71]. Patients who underwent basic CCE had a reduced incidence of acute kidney injury in the sub-acute phase of their illness [72].
CCE following cardiac arrest
CCE has been used during cardiac arrest to identify patients with pulseless electrical activity (PEA) who still have cardiac contractile activity. This can predict those who are likely to have a return of spontaneous circulation (ROSC). A recent systematic review of 11 studies included 777 patients with PEA and demonstrated that patients with cardiac activity on ultrasonography were more likely to have a ROSC. Possible added value of this ultrasonographic finding derives from the fact that it may encourage continuation of resuscitative efforts [73].
CCE in respiratory failure
Although CCE findings allow the intensivist to adapt the respiratory strategy to the patient’s RV function, the impact of this approach on prognosis has not yet been evaluated [59]. Another area in which CCE plays a key role is the multidisciplinary management of pulmonary embolism, as RV failure and shock are the main risk factors for death in this scenario. CCE plays an important role in the risk stratification and management of patients who need rapid assessment for possible reperfusion therapy [74]. Mechanical ventilation has an important influence on RV function. Increases in lung volume during inspiration and increased volume at end-expiration will increase the component of pulmonary vascular resistance resulting from increased intrathoracic pressure. Hyperinflation and increased intrathoracic pressure may impede venous return and reduce RV filling pressure. When hyperinflation increases pulmonary vascular resistance, subsequent fluid resuscitation may produce acute RV strain manifesting itself as paradoxical septal shift, and if severe, as tricuspid regurgitation. Therefore, direct assessment of RV function can help the clinician to titrate ventilatory settings in order to minimize cardiovascular dysfunction while still supporting gas exchange.
CCE in the perioperative setting
Perioperative use of CCE is another important application in daily practice. In patients undergoing major surgery who have a significant risk of major adverse cardiovascular and pulmonary events, pre- and intraoperative use of echocardiography may help the clinician to decide, often on the basis of a strategic management triage, the best strategy for reducing the risk of perioperative complications, which may consist of enhanced monitoring or a higher level of postoperative care [75, 76]. We feel that CCE outcome research focusing on the prevention of perioperative morbidity and cost effectiveness should be part of the current research agenda. Echocardiography in the critical care setting can be divided into that used for post-cardiac surgery patients and that used for patients who are undergoing major non-cardiac surgery.
Most elective cardiac surgery patients undergo a pre-operative echo, which will provide prognostic information for stratification of their risk of postoperative complications and allow application of a strategic management triage. Emergency CCE is particularly valuable for the timely diagnosis of aortic dissection (video 5 ESM) and acute valvular regurgitation [77]. Many cardiac surgery patients will have intraoperative echocardiography, thereby allowing continuity of monitoring with post-operative scanning [78]. CCE is well established as a first response tool for evaluating hemodynamic instability, low CO syndrome, post-cardiotomy acute RV failure, prosthetic valve dysfunction, unexplained severe hypoxemia, and cardiac tamponade. TEE is often favored in this group of patients, particularly for the diagnosis of regional tamponade (video 6 ESM) (Fig. 5), although the feasibility of TTE appears to improve after the first post-operative day [79,80,81]. Although there is a lack of hard outcome data, CCE is considered a routine application for the management of the pre- and postoperative cardiac surgery patient. Recently the use of a disposable 72-h TEE device, which provides more frequent monitoring, has been explored along the operative pathway and holds some promise [61, 82].
Postoperative complications are frequent in the high-risk surgical population and may include cardiorespiratory instability due to hypovolemia, hemorrhage, sepsis, acute cardiac dysfunction and pulmonary embolus. CCE is an effective imaging method for diagnosing and managing these complications. The impact of pre- and intraoperative echocardiography on patient survival in the high-risk surgical population with multiple comorbidities and/or those undergoing higher risk surgical procedures (liver transplantation, major vascular surgery, severe trauma, and refractory shock) has already been addressed in the literature [83, 84]. Integration of CCE and lung ultrasonography can be particularly valuable in the perioperative management of obstetric patients [85].
CCE for mechanical circulatory support
Both TTE and TEE are essential tools for the management of both veno-venous and veno-arterial ECMO [86, 87]. Specifically, as summarized in Table 3, they are useful for the following steps: disease assessment, insertion, maintenance, and weaning. CCE imaging is used to guide correct cannula placement in the right atrium, whenever a double-lumen cannula is used and also for serial checks of cannula position and function (Fig. 6, video 7 ESM). It is also required for the ongoing assessment of RV and LV function [88, 89]. Echocardiographic quantification of ventricular function is required when weaning from the circuit is under consideration [90, 91].
Critical care echocardiography applications during extracorporeal membrane oxygenation (ECMO) support. a Verification of guide wire/cannula peripheral insertion under transesophageal echocardiography guidance in venous-arterial ECMO support; a1 descending aorta short and long axis view with presence of intra-arterial guide wire (arrows); a2 bicaval view, presence of venous ECMO cannula inside right atria (asterisks). b Visualization of peripherally inserted venous ECMO cannula with transthoracic echocardiography (TTE) by a subcostal inferior vena cava (IVC) view with utilization of color flow Doppler. c Reposition of misplaced bicaval double-lumen catheter under TTE guidance in veno-venous ECMO support; c1 absence of ECMO cannula inside the atria; c2 appropriate advancement of ECMO cannula inside the IVC (arrow); c3 presence of color flow Doppler signal suggestive of appropriate return of oxygenated blood toward tricuspid valve
CCE: skills, training and current diplomas
Required skills
The individual skills that make up the CCE toolkit were best described in the 2009 ACCP/SRLF consensus document [3]. A straightforward way to analyze them is considering their use in the following key areas: image generation, image interpretation and clinical integration. In basic CCE, image generation requires mastery of the four core cardiac views, namely (1) parasternal long-axis, (2) parasternal short-axis, (3) apical four-chamber, and (4) subcostal four-chamber as well as a subcostal view of the IVC. Image interpretation, too, has four core elements: (1) assessment of LV size and systolic function, (2) assessment of RV size and function, (3) assessment of the pericardial space for fluid, and (4) assessment of IVC size and respiratory variation. For the integration of CCE findings into the broader clinical picture, a basic provider focuses on six clinical scenarios commonly encountered in critical care: (1) severe hypovolemia, (2) LV failure, (3) RV failure, (4) pericardial tamponade, (5) severe left-sided valvular regurgitation, and (6) the use of CCE during cardiac arrest.
The image generation/image interpretation/clinical integration framework also holds true for the advanced CCE skill set, which is used to explore complex clinical scenarios. While some of the more complex pathologies are best seen from a transesophageal approach, the ultrasonography image acquisition route (TTE vs. TEE) should be selected on the basis of a combination of factors, including severity of illness, the clinical question to be answered, and the difficulty in acquiring surface images. It is an oversimplification to state that basic CCE should be performed using TTE, while advanced CCE only entails TEE. Both are complementary and may be used to address both basic and complex questions depending on the clinical circumstances.
The level of competency in image acquisition necessary to perform advanced CCE is similar to that required of cardiologist echocardiographers, and it includes all TTE and TEE views that are standard in the performance of a complete echocardiography study. In the image interpretation of an advanced exam, the same elements considered in a basic exam are examined, but at a much higher level of complexity and detail. For example, LV assessment would begin with an assessment of ventricular size and overall systolic function, but then also include elements such as diastolic function, segmental wall motion abnormalities, and measurement of stroke volume. RV assessment would involve assessment of septal motion, estimation of pulmonary artery pressures, and Doppler assessment of RV outflow patterns. Imaging of the pericardium would go beyond detection of an effusion to an assessment for the presence of tamponade physiology.
Finally, as regards integration of CCE findings into the broader clinical picture, advanced CCE expands the scope of investigation, also considering more complex pathologies such as infective endocarditis, aortic dissection, pulmonary embolism, cardiac source of embolism, intracardiac shunt detection, cardiac trauma, and the complications of acute myocardial infarction. While different medical societies have their own individual perspectives on the specifics of the CCE skill set, guideline statements published since 2009 have largely agreed on the various objectives described, lending overall support to the package as described above [92,93,94].
Training for intensivists: the key role of simulation
In the past, guidelines in CCE education have tended to be structured around clinical cardiology training rather than critical care training, but this is now changing rapidly [95]. To attain competency in advanced CCE, one would need to undergo a training that includes the ten curricular components reported in Table 4.
Early development of the definitive technical skills required to obtain, independently, optimal echocardiographic images, and to recognize diagnostic features in critically ill patients, is essential to the learning process. Current efforts in simulation education are becoming crucial to the standardization of CCE training. Skinner et al. demonstrated the efficacy of an independent, self-study, fully portable simulator-based curriculum in training novice residents in basic CCE image acquisition and interpretation [96]. Cognitive and psychomotor skills improved after self-training in similar fashion using a simulator-driven training program [97, 98]. This distinct simulation modality enables the trainee to engage in self-directed learning at his/her own pace, and it makes provision for formative assessment with immediate feedback and reassessment of the learner’s skills. The simulator indicates the level of image acquisition accuracy by showing the maximal angle deviation, the axis of rotation relative to the underlying cardiac structures, and the location of the probe on the chest wall.
Although there is still a scarcity of data regarding the transfer of simulation-acquired skills to clinical practice, some studies have reported significant improvements in the learning curve of basic CCE [99], but also in the ability of trainees to perform a full TEE hemodynamic evaluation [100].
Current diplomas in advanced CCE available worldwide
Once accreditation by examination and log book became accepted and recognized as a model and an essential part of training in cardiology and cardiothoracic anesthesia in the USA and Europe, the way was clearly paved for critical care echo accreditation [101,102,103].
As previously described, CCE has, to date, evolved on two different levels: basic and more comprehensive or advanced. A different approach to testing competency is therefore needed for each of these levels, with the assumption being that those learning the more advanced skills have already attained competency in the basic skill set. Diplomas in advanced CCE have now been established in the United Kingdom and France through national societies [12, 104]. The European Society of Intensive Care Medicine (ESICM) has developed a pan-European certification in advanced CCE called EDEC (European Diploma in advanced critical care EchoCardiography). Although the European diplomas are similar in scope, there are considerable differences between them (Table 5). The UK diploma focuses solely on TTE as practiced in critical care; a separate UK TEE diploma exists, but this focuses largely on cardiac anesthesia and cardiology practice. The ESICM EDEC diploma, launched in 2016, requires competency in both TTE and TEE, as the ESICM echo working group regards TEE an essential part of advanced CCE. In contrast to cardiology certification such as the EACVI/EACTA TEE certification (see legend to Table 5), the EDEC certification focuses on assessment of hemodynamics in the critically ill rather than detailed valvular assessment, which remains the domain of the cardiologist and specialist anesthetist. Although created in Europe, the EDEC is informed by a broad international group of experts and attracts registrants from many countries across all continents.
In 2015, the professional critical care societies in North America reached an agreement with the National Board of Echocardiography (NBE) to develop a certification in advanced CCE. The process has taken the form of a cooperative project with the full participation of the American College of Chest Physicians, American Thoracic Society, Society of Critical Care Medicine, American Society of Anesthesiology, American Society of Anesthesiologists, Society of Cardiovascular Anesthesiologists, American College of Emergency Physicians, and American Society of Echocardiography. Each society has two representatives on the working committee that was tasked with writing the qualifying examination and establishing additional certification criteria beyond simply passing the examination. The NBE reached an agreement with the National Board of Medical Examiners (NBME) to develop the examination—a full-day board style examination held at multiple computerized testing centers throughout North America. The NBME has extensive experience in developing examinations, as it is responsible for all the major board examinations for the various medical subspecialties in the USA. The examination will be held on an annual basis. At time of writing, 508 candidates were registered to take the first examination, scheduled to take place in January 2019.
In addition to the requirement that the candidate pass the board examination, the working committee is in the process of developing the final criteria required for certification in advanced CCE. These will be finalized in cooperation with the NBE in 2019 and will be modeled on the requirements set forth in 2014 Statement on Training in advanced CCE [15] with some adaptation for local circumstances. They will include the requirement that the candidate demonstrate his/her competency in image acquisition on the basis of his/her performance of at least 150 full TTE studies under the close supervision of a capable mentor, as well as proof of regular involvement with provision of critical care services. The working group is tasked with defining the criteria for the mentorship function and drawing up more detailed criteria defining the provision of critical care services. The NBE certification will require competency in TEE image interpretation but not in TEE image acquisition. This is in recognition of the fact that TEE probes are not yet widely available to intensivists in the USA.
The future
New technologies and their applications in the critical care setting
New technologies, such as speckle tracking, appear promising [68, 70]. The use of three-dimensional (3D) echocardiography has also been described in critically ill patients, but the technique is still hampered by major limitations (Fig. 7) [105]. In particular, the use of speckle tracking and 3D imaging is limited by the fact that the ultrasonography machines currently in widespread use in critical care units do not have these capabilities. Furthermore, the added cost and complexity of the machines required may also limit the use of these interesting technologies. A major development that may lead to more widespread use of basic CCE and CCUS is the emergence of a new generation of miniaturized handheld ultrasonography machines, which are inexpensive, easy to use and provide good image quality. Some models interface with smart phones and have sophisticated internet connectivity. None yet include spectral Doppler capability, so they are not fully capable of performing advanced CCE. These handheld echocardiography devices may prove a useful tool both for experts and novices, but studies are needed to fully clarify their potential [106, 107]. As high-quality handheld devices gain acceptance, it is reasonable to expect that the use of echocardiography will spread further not only in ICUs but also in the pre-hospital, perioperative and emergency department settings. It seems likely that intensivists will acquire small, low-cost machines in the coming years. The increasingly widespread dissemination of this new technology will necessitate the development of robust training systems to ensure that they are used competently by the critical care community, because the low cost, and therefore ready availability, of the pocket-sized units carries the risk that they might be used by inadequately trained clinicians, resulting in harm to patients and bringing discredit to the field of CCUS.
The figure shows three images of speckle tracking (strain) echocardiography and one of 3D echocardiography. Two strain images are taken from apical four-chamber view (top left) and apical two-chamber view (top right). Each cardiac segment reports a percentage of strain. The deformation (strain) of the single segments of each view is plotted over time in the lower part of each image. The bottom left image summarizes all strain values of the 17 cardiac segments (abnormal values are found in the mid inferoseptal and anteroseptal segments, both 12). The bottom right image refers to a 3D apical four-chamber view
Standardization of results reporting
The heterogeneity in data reporting across CCE clinical studies prompted a panel of experts from the ESICM echocardiography working group to investigate the concept of guidelines for the reporting of CCE studies. This ongoing project, named “PRICES”, aims to provide recommendations that will allow standardized measurement and data collection, and thus enable data sharing and large-scale collaborations. The guidelines will be developed after a systematic screening of the methodology and reporting strategy of previously published CCE studies (PROSPERO registration number: CRD42018094450). The project participants intend to publish the results and recommendations in late 2019.
The research agenda
In 2017, Intensive Care Medicine published a research agenda for CCUS that included 10 proposals for studies in which CCE was featured [108]. In Table 1, we propose the main studies that would help to better characterize the role of CCE in the ICU as well as its impact on patient prognosis. We also report the main persisting uncertainties in CCE. A central aspect of any research is the reproducibility of the measures across studies and individual operators. There are perhaps a few other fields of investigation in which the quality of the data (here, the image) is, as in this case, determined more by the operator than the disease. Software technology must be developed to objectively assess image quality and quantify defined metrics, including advanced metrics, such as radial regional strain and speckle-tracking dyssynchrony. This will make it possible to build up more robust clinical data sets, both before and after interventions, that can be translated directly into clinical practice.
Conclusion
CCE is now widely accepted by the critical care community as a valuable tool in the ICU and emergency department, and in perioperative settings. It allows rapid and accurate diagnosis, and it is useful for guiding the ongoing management of the critically ill patient. Advanced CCE allows full hemodynamic monitoring, leading to adaptation of the circulatory and respiratory strategy. Structured training programs for both basic and advanced CEE have been developed in the past few years, and international level certification is now available for advanced CCE.
Change history
15 April 2019
The original version of this article unfortunately contained a mistake.
References
Cholley B, Vieillard-Baron A, Mebaaza A (2006) Echocardiography in the ICU: time for widespread use! Intensive Care Med 32:9
Levitov A, Frankel HL, Blaivas M et al (2016) Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-Part II: cardiac ultrasonography. Crit Care Med 44:1206–1227
Mayo PH, Beaulieu Y, Doelken P et al (2009) American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 135:1050–1060
Papolos A, Narula J, Bavishi C, Chaudhry F, Sengupta P (2016) U.S. hospital use of echocardiography. Insights from the Nationwide Inpatient Sample. J Am Coll Cardiol 67:502–511
Dres M, Austin PC, Pham T, Aegerter P, Guidet B, Demoule A, Vieillard-Baron A, Brochard L, Geri G, CUB-REA Group (2018) Acute respiratory distress syndrome cases volume and ICU mortality in medical patients. Crit Care Med 46:e33–e40
De Backer D, Bakker J, Cecconi M, Hajjar L, Liu DW, Lobo S, Monnet X, Morelli A, Myatra SN, Perel A, Pinsky MR, Saugel B, Teboul JL, Vincent JL (2018) Alternatives to the Swan-Ganz catheter. Intensive Care Med 44:730–741
Mercado P, Maizel J, Beyls C, Kontar L, Orde S, Huang S, McLean A, Tribouilloy C, Slama M (2019) Reassessment of the accuracy of cardiac Doppler pulmonary artery pressure measurements in ventilated ICU patients: a simultaneous Doppler-catheterization study. Crit Care Med 47:41–48
Zieleskiewicz L, Muller L, Lakhal K et al (2015) Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med 41:1638–1647
Vincent JL, Rhodes A, Perel A et al (2011) Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care 15:229
Cecconi M, Hofer C, Teboul JL et al (2015) Fluid challenge in intensive care: the FENICE study. A global inception cohort study. Intensive Care Med 41:1529–1537
Jensen MB, Sloth E, Larsen KM, Schmidt MB (2004) Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol 21:700–707
Fletcher SN, Grounds RM (2012) Critical care echocardiography: cleared for take up. Br J Anaesth 109:490–492
Price S, Via G, Sloth E, Guarracino F, Breitkreutz R, Catena E, Talmor D (2008) Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound 6:49
Neskovic AN, Skinner H, Price S et al (2018) Focus cardiac ultrasound core curriculum and core syllabus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 19:475–481
Expert round table on echocardiography in ICU (2014) International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med 40:654–666
Cook CH, Praba AC, Beery PR, Martin LC (2002) Transthoracic echocardiography is not cost-effective in critically ill surgical patients. J Trauma 52:280–284
Lhéritier G, Legras A, Caille A, Lherm T, Mathonnet A, Frat JP, Courte A, Martin- Lefèvre L, Gouëllo JP, Amiel JB, Garot D, Vignon P (2013) Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med 39:1734–1742
Garcia YA, Quintero L, Singh K, Lakticova V, Lakovou A, Koenig SJ, Narasimhan M, Mayo PH (2017) Feasibility, safety, and utility of advanced critical care transesophageal echocardiography performed by pulmonary/critical care fellows in a medical ICU. Chest 152:736–741
Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K (2004) The use and safety of transesophageal echocardiography in the general ICU-a minireview. Acta Anaesthesiol Scand 48:827–836
Orde S, Slama M, Hilton A, Yastrebov K, McLean A (2017) Pearls and pitfalls in comprehensive critical care echocardiography. Crit Care 21:279
Panoulas VF, Daigeler AL, Malaweera AS, Lota AS, Baskaran D, Rahman S, Nihoyannopoulos P (2013) Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging 14:323–330
Martin LD, Howell EE, Ziegelstein RC, Martire C, Whiting-O’Keefe QE, Shapiro EP, Hellmann DB (2009) Hand-carried ultrasound performed by hospitalists: does it improve the cardiac physical examination? Am J Med 122:35–41
Di Bello V, La Carrubba S, Conte L et al (2015) Incremental value of pocket-sized echocardiography in addition to physical examination during inpatient cardiology evaluation: a multicenter Italian study (SIEC). Echocardiography 32:1463–1470
Mjolstad OC, Dalen H, Graven T, Kleinau JO, Salvesen O, Haugen BO (2012) Routinely adding ultrasound examinations by pocket-sized ultrasound devices improves inpatient diagnostics in a medical department. Eur J Intern Med 23:185–191
Hibbert B, Simard T, Ramirez FD (2018) Impact of routine handheld focused cardiac ultrasonography on the diagnosis and management of hospitalized cardiac patients: the CAPITAL FoCUS Registry. https://www.ahajournals.org/doi/abs/10.1161/circ.134.suppl_1.20662. Accessed 10 Oct 2018
Vignon P, Mentec P, Terré S, Gastinne H, Guéret P, Lemaire F (1994) Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 106:1829–1834
Heidenreich PA, Stainback RF, Redberg RF, Schiller NB, Cohen NH, Foster E (1995) Transesophageal echocardiography predicts mortality in critically ill patients with unexplained hypotension. J Am Coll Cardiol 26:152–158
McKaigney CJ, Krantz MJ, La Rocque CL, Hurst ND, Buchanan MS, Kendall JL (2014) E-point septal separation: a bedside tool for emergency physician assessment of left ventricular ejection fraction. Am J Emerg Med 32:493–497
Nazerian P, Vanni S, Zanobetti M, Polidori G, Pepe G, Federico R, Cangioli E, Grifoni S (2010) Diagnostic accuracy of emergency Doppler echocardiography for identification of acute left ventricular heart failure in patients with acute dyspnea: comparison with Boston criteria and N-terminal prohormone brain natriuretic peptide. Acad Emerg Med 17:18–26
Kimura BJ, Amundson SA, Willis CL, Gilpin EA, DeMaria AN (2002) Usefulness of a hand-held ultrasound device for bedside examination of left ventricular function. Am J Cardiol 90:1038–1039
Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA (2002) Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med 9:186–193
Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC (2016) Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound 44:92–99
Vignon P, Mucke F, Bellec F, Marin B, Croce J, Brouqui T, Palobart C, Senges P, Truffy C, Wachmann A, Dugard A, Amiel J (2011) Basic critical care echocardiography: validation of a curriculum dedicated to noncardiologist residents. Crit Care Med 39:636–642
Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO (2001) Bedside echocardiography by emergency physicians. Ann Emerg Med 38:377–382
Mekontso-Dessap A, Chew MS (2018) Cardiac tamponade. Intensive Care Med 44:936–939
Douglas PS, Garcia MJ, Haines DE et al (2011) ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. J Am Coll Cardiol 57:1126–1166
Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D (2014) Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med 21:843–852
Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, deSouza I, Sinert R (2016) Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med 23:223–242
Pivetta E, Goffi A, Lupia E et al (2015) Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest 148:202–210
Sanfilippo F, Scoletta S, Morelli A, Vieillard-Baron A (2018) Practical approach to diastolic function in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care 8:100
Clancy DJ, Scully T, Slama M, Huang S, McLean A, Orde SR (2017) Application of updated guidelines on diastolic dysfunction in patients with severe sepsis and septic shock. Ann Intensive Care 7:121
Vieillard-Baron A, Naeije R, Haddad F (2018) Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med 44:774–790
Mekontso-Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, Brun- Buisson C, Vignon P, Vieillard-Baron A (2016) Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 42:862–70
Plummer D, Brunette D, Asinger R, Ruiz E (1992) Emergency department echocardiography improves outcome in penetrating cardiac injury. Ann Emerg Med 21:709–712
Ferrada P, Evans D, Wolfe L, Anand RJ, Vanguri P, Mayglothling J, Whelan J, Malhotra A, Goldberg S, Duane T, Aboutanos M, Ivatury RR (2014) Findings of a randomized controlled trial using limited transthoracic echocardiogram (LTTE) as a hemodynamic monitoring tool in the trauma bay. J Trauma Acute Care Surg 76:31–37
Gaspari R, Weekes A, Adhikari S et al (2016) Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 109:33–39
Jones AE, Tayal VS, Sullivan DM, Kline JA (2004) Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med 32:1703–1708
Joseph MX, Disney PJ, Da Costa R, Hutchison SJ (2004) Transthoracic echocardiography to identify or exclude cardiac cause of shock. Chest 126:1592–1597
Wetterslev M, Moller-Sorensen H, Johansen RR, Perner A (2016) Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 42:1223–1233
Pinsky MR, Payen D (2005) Functional hemodynamic monitoring. Crit Care 9:566–572
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis. Bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, Slama M, Prat G, Vieillard-Baron A (2017) Comparison of echocardiography indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 195:1022–1032
Vieillard-Baron A, Evrard B, Repessé X, Maizel J, Jacob C, Goudelin M, Charron C, Prat G, Slama M, Geri G, Vignon P (2018) Limited value of end-expiratory inferior vena cava diameter to predict fluid responsiveness. Impact of intra-abdominal pressure. Intensive Care Med 44:197–203
Repessé X, Charron C, Vieillard-Baron A (2016) Assessment of the effects of inspiratory load on right ventricular function. Curr Opinion Crit Care 22:254–259
Mahmood SS, Pinsky MR (2018) Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med 6:349
Magder S (2018) Heart-lung interactions in spontaneous breathing subjects: the basics. Ann Transl Med 6:348
Vignon P, Begot E, Mari A et al (2018) Hemodynamic assessment of patients with septic shock using transpulmonary thermodilution and critical care echocardiography: a comparative study. Chest 153:55–64
Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Madger S, Marini JJ (2016) Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 42:739–749
Repessé X, Charron C, Vieillard-Baron A (2015) Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest 147:259–265
Vieillard-Baron A, Slama M, Mayo P, Charron C, Amiel JB, Esterez C, Leleu F, Repesse X, Vignon P (2013) A pilot study on safety and clinical utility of a single-use 72-hours indwelling transesophageal echocardiography probe. Intensive Care Med 39:629
Fletcher N, Geisen M, Meeran H, Spray D, Cecconi M (2015) Initial clinical experience with a miniaturized transesophageal probe in a cardiac intensive care unit. J Cardiothorac Vasc Anesth 29:582–587
Cioccari L, Baur HR, Berger D, Wiegand J, Takala J, Merz TM (2013) Hemodynamic assessment of critically ill patients using a miniaturized transesophageal echocardiography probe. Crit Care 17:R121
Manasia AR, Nagaraj HM, Kodali RB (2005) Feasibility and potential clinical utility of goal-directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand-carried device (SonoHeart) in critically ill patients. J Cardiothorac Vasc Anesth 19:155–159
Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevitch V, Brown DR, Oh JK (2012) Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc 87:620–628
Huang SJ, Nalos M, McLean AS (2013) Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care 17:R96
Guarracino F, Baldassarri R, Pinsky MR (2013) Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 17:213
Huang SJ, Ting I, Huang AM, Slama M, McLean AS (2017) Longitudinal wall fractional shortening: an M-mode index based on mitral annular plane systolic excursion (MAPSE) that correlates and predicts left ventricular longitudinal strain (LVLS) in intensive care patients. Crit Care 21:292
Orde SR, Pulido JN, Masaki M, Gillespie S, Spoon JN, Kane GC, Oh JK (2014) Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care 18:R149
Boissier F, Razazi K, Seemann A, Bedet A, Thille AW, de Prost N, Lim P, Brun-Buisson C, Mekontso-Dessap A (2017) Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 43:633–642
Sanfilippo F, Corredor C, Fletcher N, Tritapepe L, Lorini FL, Arcadipane A, Vieillard- Baron A, Cecconi M (2018) Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: a systematic review and meta-analysis. Crit Care 22:183
Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, Vieillard- Baron A, Celi L (2018) Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 44:884–892
Kanji HD, McCallum J, Sirounis D, MacRedmond R, Moss R, Boyd JH (2014) Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care 29:700–705
Wu C, Zheng Z, Jiang L, Gao Y, Xu J, Jin X, Chen Q, Zhang M (2018) The predictive value of bedside ultrasound to restore spontaneous circulation in patients with pulseless electrical activity: a systematic review and meta-analysis. PLoS One 13:e0191636
Konstantinides SV, Torbicki A, Agnelli G et al (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35:3033–3069
Cowie B (2002) Focused transthoracic echocardiography predicts perioperative cardiovascular morbidity. J Cardiothorac Vasc Anesth 26:989–993
Canty DJ, Heiberg J, Yang Y, Royse AG, Margale S, Nanjappa N, Scott D, Maier A, Sessler DI, Chuan A, Palmer A, Bucknill A, French C, Royse CF (2018) Pilot multi-centre randomised trial of the impact of pre-operative focused cardiac ultrasound on mortality and morbidity in patients having surgery for femoral neck fractures (ECHONOF-2 pilot). Anaesthesia 73:428–437
Lancellotti P, Price S, Edvardsen T et al (2015) The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 16:119–464
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH (2014) Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg 118:21–68
Heiberg J, El-Ansary D, Royse CF, Royse AG, Alsaddique AA, Canty DJ (2016) Transthoracic and transoesophageal echocardiography: a systematic review of feasibility and impact on diagnosis, management and outcome after cardiac surgery. Anaesthesia 71:1210–1221
Canty DJ, Heiberg J, Tan JA, Yang Y, Royse AG, Royse CF, Mobeirek A, Shaer FE, Albacker T, Nazer RI, Fouda M, Bakir BM, Alsaddique AA (2017) Assessment of image quality of repeated limited transthoracic echocardiography after cardiac surgery. J Cardiothorac Vasc Anesth 31:965–972
Geisen M, Spray D, Nicholas Fletcher S (2014) Echocardiography-based hemodynamic management in the cardiac surgical intensive care unit. J Cardiothorac Vasc Anesth 28:733–744
Treskatsch S, Balzer F, Knebel F, Habicher M, Braun JP, Kastrup M, Grubitzsch H, Wernecke KD, Spies C, Sander M (2015) Feasibility and influence of hTEE monitoring on postoperative management in cardiac surgery patients. Int J Cardiovasc Imaging 31:1327–1335
Jasudavisius A, Arellano R, Martin J, McConnell B, Bainbridge D (2016) A systematic review of transthoracic and transesophageal echocardiography in non-cardiac surgery: implications for point-of-care ultrasound education in the operating room. Can J Anaesth 63:480–487
Staudt GE, Shelton K (2018) Development of a rescue echocardiography protocol for noncardiac surgery patients. Anesth Analg. https://doi.org/10.1213/ane.0000000000003569
Zieleskiewicz L, Bouvet L, Einav S, Duclos G, Leone M (2018) Diagnostic point-of-care ultrasound: applications in obstetric anaesthetic management. Anaesthesia 73:1265–1279
Victor K, Barrett NA, Gillon S, Gowland A, Meadows CI, Ioannou N (2015) Critical care echo rounds: extracorporeal membrane oxygenation. Echo Res Pract 2:D1–D11
Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF (2012) The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr 25:131–141
Douflé G, Roscoe A, Bilia F, Fan E (2015) Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 19:326
Donker DW, Meuwese CL, Braithwaite SA, Broomé M, van der Heijden JJ, Hermens JA, Platenkamp M, de Jong M, Janssen JGD, Balík M, Bělohlávek J (2018) Echocardiography in extracorporeal life support: a key player in procedural guidance, tailoring and monitoring. Perfusion 33:31–41
Aissaoui N, Caudron J, Leprince P, Fagon JY, Lebreton G, Combes A, Diebold B (2017) Right-left ventricular interdependence: a promising predictor of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 43:592–594
Aissaoui N, Luyt CE, Leprince P, Trouillet JL, Léger P, Pavie A, Diebold B, Chastre J, Combes A (2011) Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 37:1738–1745
Arntfield RT, Millington SJ, Ainsworth CD et al (2014) Canadian recommendations for critical care ultrasound training and competency. Can Respir J 21:341–345
Spencer KT, Kimura BJ, Korcarz CE (2013) Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 26:567–581
Expert Round Table on Ultrasound in ICU (2011) International expert statement on training standards for critical care ultrasonography. Intensive Care Med 37:1077–1083
Ryan T, Berlacher K, Lindner JR et al (2015) COCATS 4 task force 5: training in echocardiography. J Am Coll Cardiol 65:1786–1799
Skinner AA, Freeman RV, Sheehan FH (2016) Quantitative feedback facilitates acquisition of skills in focused cardiac ultrasound. Sim Healthcare 11:134–138
Ferrero NA, Bortsov AV, Arora H et al (2014) Simulator training enhances resident performance in transesophageal echocardiography. Anesthesiology 120:149–159
Damp J, Anthony R, Davidson MA, Mendes L (2013) Effects of transesophageal echocardiography simulator training on learning and performance in cardiovascular medicine fellows. J Am Soc Echocardiogr 26:1450–1456.e2
Vignon P, Pegot B, Dalmay F, Jean-Michel V, Bocher S, L’her E, Cros J, Prat G, EchoSimu Group (2018) Acceleration of the learning curve for mastering basic critical care echocardiography using computerized simulation. Intensive Care Med 44:1097–1105
Prat G, Charron C, Repesse X, Coriat P, Bailly P, L’her Vieillard-Baron E, Vieillard-Baron A A (2016) The use of computerized echocardiographic simulation improves the learning curve for transesophageal hemodynamic assessment in critically ill patients. Ann Intensive Care 6:27
Aronson S, Thys DM (2001) Training and certification in perioperative transesophageal echocardiography: a historical perspective. Anesth Analg 93:1422–1427
Swanevelder J, Chin D, Kneeshaw J, Chambers J, Bennett S, Smith D, Nihoyannopoulos P (2003) Accreditation in transesophageal echocardiography: statement from the Association of Cardiothoracic Anaesthetists and the British Society of Echocardiography Joint TOE Accreditation Committee. Br J Anaesth 91:469–472
Fox KF, Popescu BA, Janiszewski S, Nihoyannopoulos P, Fraser AG, Pinto FJ (2007) Report on the European Association of Echocardiography accreditations in echocardiography: December 2003-September 2006. Eur J Echocardiogr 8:74–79. http://www.uvsq.fr/diu-techniques-ultrasoniques-en-anesthesie-et-en-reanimation-148919.kjsp
http://www.uvsq.fr/diu-techniques-ultrasoniques-en-anesthesie-et-en-reanimation-148919.kjsp
Orde S, Slama M, Stanley N, Huang S, McLean A (2018) Feasibility of biventricular 3D transthoracic echocardiography in the critically ill and comparison with conventional parameters. Crit Care 22:198
Galusko V, Bodger O, Ionescu A (2018) A systematic review of pocket-sized imaging devices: small and mighty? Echo Res Pract 5:113–138
Goudelin M, Evrard B, Dalmay F, Padilla AH, Gonzalez C, Lafon T, Daix T, Fedould AL, François B, Vignon P (2018) Diagnostic capability of a next-generation, ultra- miniaturized ultrasound system in patients with cardiopulmonary compromise assessed using basic critical care echocardiography. Intensive Care Med 44:1579–1581
Mayo P, Arntfield R, Balik M, Kory P, Mathis G, Schmidt G, Slama M, Volpicelli G, Xirouchaki N, McLean A, Vieillard-Baron A (2017) The ICM research agenda on critical care ultrasonography. Intensive Care Med 43:1257–1269
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
AVB has received grant funds from GSK for conducting clinical research and was a member of the scientific advisory board for the study. SJM declares no conflict of interest. FSF declares no conflict of interest. MC has received honoraria and travel grants from Edwards Lifesciences. JDG declares no conflict of interest. AML declares no conflict of interest. MRP has received honoraria for lectures from Edwards Lifesciences, Cheetah Medical and LiDCO Ltd and is a scientific advisor to Edwards Lifesciences and LiDCO Ltd. JP declares no conflict of interest. PM declares no conflict of interest. NF declares no conflict of interest.
Ethical approval
An approval by an ethics committee was not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the spelling of the name from F. Sanfilippo was incorrect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 14652 kb)
Supplementary material 6 (AVI 11076 kb)
Rights and permissions
About this article
Cite this article
Vieillard-Baron, A., Millington, S.J., Sanfilippo, F. et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 45, 770–788 (2019). https://doi.org/10.1007/s00134-019-05604-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05604-2