Abstract
Background
Multiple organ dysfunction syndrome is the commonest reason for sepsis-associated mortality.
Discussion
In the 40 years since it was first described understanding of its pathophysiology has improved, and novel methodologies for monitoring and severity of illness scoring have emerged. These, together with the development of systematic strategies for managing organ dysfunction in sepsis, and potentially effective new therapeutic interventions, should assist in reducing sepsis-associated mortality.
Conclusion
These historical developments are discussed, and the reader is directed to these references for further guidance.
Similar content being viewed by others
Introduction
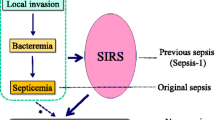
Sepsis: historical perspective
The term sepsis is derived from the Greek word sepsin, which means ‘to make putrid’. Early descriptions of disease mediated by “small invisible creatures” were made in the second century B.C., and the concepts of contagion and isolation of diseased individuals followed. Despite attempts at prevention pan-epidemic infections have caused the deaths of millions of persons throughout history. The first documented observations of living bacteria were made by van Leeuwenhoek in 1674 and classification of bacterial morphology in the early nineteenth century. However, the relationship between infectious disease, its aetiology, and its pathogenesis remained elusive.
The principles of disinfection and anti-septic practices pioneered by Semmelweis and later by Lister were adopted only several decades later. The importance of the host response to infection was first described in the 1880s and classified separately in terms of cell-mediated and humoral immunity. The subsequent use of drug anti-metabolites to ameliorate the effects of syphilis at the turn of the century, and the discovery of antibiotic sulphonamides, moulds and vaccination, led to a revolution in the treatment and prevention of infection. It was believed that these developments had the potential of eradicating sepsis from the modern age, but the problems of changing disease patterns and antibiotic resistance dampened early ambitions and sepsis has remained a formidable problem in many areas of medical practice.
Sepsis and multiple organ dysfunction: epidemiology
Sepsis, the host response to an infectious process, is termed severe when complicated by predefined organ system dysfunction [1]. Together, the systemic inflammatory response syndrome (SIRS), sepsis and septic shock have been termed the ‘sepsis syndromes’ [1, 2, 3]. The nature of infectious organisms associated with sepsis is changing. Thus, whilst Gram-negative bacteria were traditionally responsible for the majority of hospital-acquired infections, Gram-positive organisms (30–50% of cases) and multidrug-resistant bacteria or fungi (25%) are now more common [4, 5]. Moreover, the burden of sepsis-related disease is also rising; from 82.7 to 240.4 cases per 100,000 population in the United States and to 51 cases per 100,000 population (1997 figures) in the United Kingdom, where 27.1% of adult ICU admissions had severe sepsis in the first 24 h [6].
Severe sepsis and shock are characterised by tissue hypoperfusion, cellular hypoxia and metabolic dysfunction. Consequently the majority of patients with SIRS and its sequelae who fail to survive succumb to multiple organ dysfunction syndrome (MODS). Multiple organ failure (i.e. demonstrable failure of two or more organs) within the ICU was first documented in 1977. Bacterial sepsis was aetiologically significant in 69% of the cases described [7]. Indeed, the onset of MODS, synonymous with multiple organ system failure, was thought originally to follow a temporal sequence (lung, liver, gastric mucosa and kidney) [8]. Moreover, whilst strongly linked to uncontrolled infection (in particular intra-abdominal), it is now recognised that MODS can occur independently of sepsis. The commonest manifestation of MODS is acute lung injury, defined by refractory hypoxaemia attributable to high permeability pulmonary oedema [9]. Its extreme manifestation, the acute respiratory distress syndrome (ARDS), occurs in more than 40% of patients with sepsis and severe sepsis [6, 10].
There has been an evolution in the appreciation of mechanisms that result in sepsis and subsequent MODS. Thus, initially, a link between infections, which were recognised and treated and their inflammatory consequences was not appreciated. Indeed, progression to MODS, in spite of evidence of clearing of infection, nurtured the hypothesis of the body's response to infection associated systemic inflammation (by now autonomous from the initial infection) as being crucial to outcome. The process of increasing understanding of sepsis-associated MODS has required a number of key components, namely: (a) defining the biophysiological pathways arising from a systemic inflammatory insult, (b) clear epidemiological definitions of the spectrum of sepsis syndromes (often misused terms), (c) understanding the pathophysiological processes of the clinically apparent systemic disturbances during early and later stages and (d) testing different therapeutic approaches, directed at specific implicated inflammatory markers or at abnormal physiological parameters. Many therapeutic ‘bedside’ approaches have been proven wrong, yet providing insights into further ‘bench’ studies.
In summary, the sepsis syndromes and their sequelae, specifically MODS, represent the leading cause of death in adult general ICUs, with an associated mortality of 30–45%, consumption of 45% of ICU and 33% of hospital bed days and an estimated cost of $16.7 billion [6, 11].
Pathophysiology of MODS in sepsis
It is unknown why sepsis progresses to MODS in only certain individuals, or what the exact pathway is that leads to this. If the inflammatory process that characterises the systemic response to infectious pathogens becomes self-sustaining and progressive, organ dysfunction ensues. An extraordinarily complex and intricate cascade of inflammatory mediators, extra- and intracellular cell signalling pathways is activated. Prevailing wisdom suggests that these result in either microvascular dysregulation and/or mitochondrial dysfunction (so-called cytopathic hypoxia). These processes result in tissue hypoperfusion, and a further cascade of biochemico-physical alterations culminating in MODS [12].
Microvascular dysfunction
Early in the course of sepsis cardiac output (CO) rises to maintain blood pressure and organ perfusion in the face of reduced peripheral vascular resistance (hyperdynamic sepsis). As sepsis progresses, cardiac output is frequently reduced (so-called hypodynamic sepsis), which has a poor prognosis. Cardiac dysfunction per se is apparent in up to 44% of critically ill septic patients, with the aetiological agents suspected to be circulating depressant factors. Myocardial function tends to recover in survivors, and the prognostic significance of dysfunction in sepsis remains debatable [13]. Redistribution of capillary blood flow has been demonstrated in both animal models and in clinical sepsis [14, 15]. The use of investigatory tools such as intravital videomicroscopy, now applicable in the clinical setting, has provided evidence of simultaneous structural and functional abnormalities in sepsis, strengthening the association between tissue hypoperfusion and organ dysfunction. However, contradictory evidence from animal studies suggests that such hypoperfusion does not invariably lead to organ dysfunction and death.
Cytopathic hypoxia
Elevated tissue oxygen levels have been demonstrated in animals during experimental sepsis and in human skeletal muscle, suggesting that cellular inefficiency of oxygen utilisation rather than a failure of oxygen delivery (DO2) to tissues occurs in sepsis. By contrast, in cardiogenic shock tissue oxygen is reduced [16, 17]. Tissue oxygen consumption occurs normally principally through ATP production by oxidative phosphorylation in mitochondria. Reduced ATP concentrations in skeletal muscle during sepsis are associated with increasing severity of, and poor outcome from, septic shock [18]. The pathophysiological consequences of both regional flow alterations and mitochondrial dysfunction undoubtedly co-exist in the septic state, but do not appear to lead to significant histopathological correlates detectable at post-mortem examination.
Inflammatory cytokines in sepsis
The development of sequential organ failure in critically ill patients with sepsis is strongly predictive of mortality. However, the mechanisms involved in the dynamic interaction between different organ systems are dictated by the intricate interplay of haemodynamics, oxygen transport and metabolic disturbances. Genetic predisposition is almost certainly relevant in upregulating the expression of inflammatory mediators [e.g. tumour necrosis factor (TNF), interleukin (IL) 1, IL-8, triggering receptor on myeloid cells 1, high mobility group box 1), thereby influencing adversely the anti-/pro-inflammatory balance. Genetic predisposition seems more important for some infectious diseases such as meningococcaemia, but polymorphisms such as for TNF-α gene promoter can play a more general role in susceptibility to septic shock associated mortality [19]. Neuroendocrine systems and prothrombotic pathways (e.g. tissue factor) are activated with downregulation of fibrinolytic systems (i.e. anti-thrombin III, activated protein C and tissue factor pathway inhibitor) [20]. Inflammatory mediators TNF, IL-1, nitric oxide and reactive oxygen species are believed to disrupt communication pathways between organs which precedes organ failure [21]. Indeed, epithelial dysfunction has been proposed as a final common pathway for organ dysfunction in sepsis [22]. The tight junctions between these cells are affected in experimental models of sepsis. This may be particularly relevant in the gastrointestinal tract, which has been variously proposed as the ‘seat of sepsis’ and the ‘motor of multiple organ failure’ [23, 24]. Bacterial translocation (i.e. direct transcellular transport of microbes from the enterocytes to the submucosal layer) across a permeable intestinal luminal mucosa into the splanchnic circulation has been proposed as the initiator and propagator of sepsis following a remote insult. Mechanisms for this mucosal injury are multifactorial, including reduced intestinal blood flow and tissue hypoxia. Impaired hepatic clearance of toxins may also be relevant [25, 26, 27].
The prevailing theories of sepsis as an uncontrolled inflammatory response, which have been based on extensive animal studies, do not necessarily reflect the human clinical pattern. They used relatively large doses of bacteria or endotoxin and mortality was therefore the result of a ‘cytokine storm’, that if blocked improved survival. Meningococcaemia is perhaps the only human form of sepsis in which circulating levels of TNF-α are high and correlated with mortality [28]. Furthermore, there is much evidence of immune suppression during sepsis. Anergy (a state of non-responsiveness to antigen) through lymphocyte apoptosis has been demonstrable in vivo, and from autopsy studies of patients dying from sepsis [29]. Cellular hibernation or ‘stunning’ as occurs during myocardial ischaemia has been postulated as a mechanism for sepsis-associated MODS based on the notable findings of discordance between histological findings and the degree of organ dysfunction from patients who died of sepsis [30].
An emerging concept is the variable immune response during sepsis; from hyperimmune to hypoimmune, depending on factors that include virulence of the organism, size of the inoculum, pre-existing co-morbidity, genetic polymorphisms in candidate genes and the inflammatory insults during the course of sepsis. Therefore it is perhaps too simplistic to consider an overactive immune system as the reason for sepsis and associated MODS but rather a dynamic state where a severely compromised immune system might prevent adequate eradication of pathogens [29].
Clinical relevance of organ dysfunction: severity of illness scoring systems
Scoring systems as risk prediction tools rely on acute derangements in acute physiological parameters which are numerically assigned by degree and aggregated. Such generic (as distinct from disease-specific) scoring systems are best exemplified by the Acute Physiology and Chronic Health Evaluation (APACHE) system [31] which has led to the development of a number of other organ-based failure scores [32, 33, 34, 35].
Perhaps the most widely applied in current practice is the Sequential Organ Failure Assessment Score (SOFA, previously called the Sepsis-Related Organ Failure Assessment). Daily SOFA scores provide an important physiological tracking system for the dynamic course of critically ill patients with sepsis. Whilst not designed for mortality prediction, worse scores are strongly associated with mortality [36]; the mean and highest SOFA scores are predictors of poor prognosis, whilst a worsening of SOFA within the first 48 h predicts the likelihood of mortality 50% or higher [37]. However, whether organ-based scoring systems direct the timing, degree and duration of appropriate interventions to prevent MODS in sepsis is uncertain.
Detecting organ dysfunction in sepsis
Continuous monitoring of clinical and physiological variables, recognition of the significance of any changes in monitored parameters, and an appropriate response, are the cornerstones and defining characteristic of modern-day intensive care medicine. Electrocardiographic, peripheral temperature (as an indicator of shock or its response) [38], non-invasive oxygen saturations [39], arterial blood gas, end tidal CO2, metabolism (i.e. lactate), central venous, and cardiac output monitoring have become routine in practice. Specific organ system monitoring can guide management in certain circumstances such as intracranial pressure monitoring in traumatic head injury [40], whilst other more novel techniques such as gastric tonometry, and hepatic blood flow devices are under evaluation in the setting of sepsis [41].
Metabolic monitoring
Hyperlactataemia is multifactorial in origin. Nevertheless, there is a good relationship in sepsis between lactic acidosis, organ failure and poor outcome [42]. Indeed, blood lactate sampling is established and now recommended as an important parameter for monitoring in international guidelines on the management of severe sepsis [43].
Cardiac output monitoring
The history of the development of flow-directed, balloon-tipped, pulmonary artery catheters (PAC) saw them adopt a pivotal role in continuous bedside cardiopulmonary monitoring, and coincidently propagated the value of central venous catheters [44, 45, 46]. However, the SUPPORT [47] investigators identified an increased odds ratio for mortality and resource utilisation with the use of the PAC, even after adjustment for treatment selection bias. The ‘attributable’ morbidity associated with PAC use was thought more likely due to misinterpretation of the values thereby derived than to physical complications on insertion [48]. However, such work has led to the development of a number of other monitoring devices utilising arterial waveform analysis (i.e. pulse contour cardiac output, lithium dilution cardiac output), oesophageal Doppler and bioimpedance. Whilst all are relatively less invasive than the PAC, none provides the additional information about the pulmonary circulation. By contrast, the use of echocardiography is becoming more widespread in assessing cardiac function in sepsis [49, 50, 51, 52].
Mixed venous oxygen saturation
The value of reduced mixed venous oxygen tensions/saturations sampled from indwelling PACs as an accurate reflection of inadequate DO2 due to reduced CO in cardiorespiratory failure was first demonstrated in patients undergoing cardiac surgery in whom a close correlation between venous oxygen saturation, CO and outcome was demonstrated [53]. Central venous oxygen saturation is now regarded as a crucial physiological surrogate for identifying and directing the correction of ‘hidden’ oxygen debt [54, 55, 56].
Management of organ dysfunction in sepsis
The principles of management of severe sepsis and associated organ dysfunction have evolved concomitantly with an increasing evidence base. Some critical concepts and studies that have helped this development are discussed below.
Diagnosis, source control and anti-microbial therapy
Early diagnosis of infection, ‘source control’ and appropriate anti-microbial treatment have been reported as crucial to outcome in sepsis for many years [57]. By contrast, up to eight-fold higher mortality is observed in prospective cohort studies of antibiotic misuse [58, 59], while inadequate surgical source control predicts MODS and increases mortality [7, 60].
Resuscitation-fluid management
Prompt and adequate haemodynamic resuscitation in patients with severe sepsis is pivotal in preventing progression to MODS and death. International recommendations suggest achieving a central venous pressure of 8–12 mmHg (or 12–15 mmHg in mechanically ventilated patients [56]. Which type of fluid replacement (i.e. crystalloid vs. colloid or albumin) to administer is more contentious [61, 62, 63], although a recent position statement by the American Thoracic Society is helpful in this regards [64].
Haemodynamic goals in sepsis
Fluid resuscitation in septic shock is directed at achieving adequate tissue perfusion and oxygenation, thereby overcoming tissue oxygen ‘debt’ which relates in part to inadequate DO2. However, an early demonstration that dobutamine and adequate volume resuscitation improve DO2 (and oxygen consumption, VO2) as well as haemodynamic parameters post-operatively [65, 66, 67] was not reproduced in patients with sepsis-induced organ failures. Indeed, a strategy of goal directed supranormal oxygen delivery (cardiac index 4.5 l min−1 m−2, DO2 > 60 ml min−1 m−2, VO2 > 170 ml min−1 m−2) using dobutamine in volume resuscitated critically ill patients increased mortality (54%) compared to controls (34%) [68]. In fact, the dobutamine-‘driven’ patients did not increase their VO2 beyond those of adequately volume resuscitated controls. A second study with similar outcomes [69] helped to establish a number of facts. First, patients with sepsis and septic shock who can improve their haemodynamic indices through adequate fluid resuscitation are likely to do better than those who do not. Second, supranormal targets for DO2/VO2 are at best unnecessary, and at worst increase mortality. Third, a beneficial response to fluid resuscitation is more likely in the acute phase, before established critical illness. Thus patients with severe sepsis and septic shock resuscitated to standard haemodynamic goals, who additionally achieve central venous oxygen saturation of 70% or higher within the first 6 h by fluid resuscitation, red cell transfusion to a haematocrit of 30%, and/or dobutamine (up to 20 μg kg−1 min−1) display significantly lower 30- and 60-day mortality rates [56].
Ventilatory strategies
In those patients with sepsis who develop acute lung injury and require mechanical ventilatory support low tidal volumes (approx. 6 ml/kg) and inspiratory plateau pressures below 30 cmH2O should be used where possible. Such recommendations have emerged from animal studies [70, 71] and a retrospective analysis of patients with ARDS, which demonstrated that pressure-limited ventilation with so-called permissive hypercapnia reduced hospital mortality compared with APACHE II predictions (18.6% vs. 37.8%) [72]. It was, however, the pivotal ARDSnet study that demonstrated a 9% absolute mortality reduction (31% vs. 39.8% for controls) in patients with ARDS randomised to receive a tidal volume of 6 ml/kg with plateau pressure limited to less than 30 cmH2O [73]. By contrast, higher positive end expiratory pressures, prone positioning and the use of inhaled nitric oxide and surfactant have demonstrated only short-term improvements in oxygenation. The results of a large randomised controlled trial of steroid therapy in late stage ARDS based upon an encouraging single-centre study are awaited [74].
Management of renal dysfunction
The importance of maintaining regional perfusion in sepsis is increasingly recognised, not least the hepatosplanchnic circulation. Since the first experiences of arteriovenous haemofiltration in anuric intensive care patients with fluid overload resistant to diuretics in the 1970s [75], acute renal failure in the critically ill has been recognised to be of multifactorial aetiology. Hypotension, nephrotoxic drug insults, sepsis and preceding renal dysfunction may all be relevant [76]. Acute renal failure is an independent risk factor for mortality in the critically ill, which varies from 45% to 70% when associated with sepsis [77, 78]. Factors predicting a poor outcome are advanced age, altered previous health status, later onset of acute renal failure, sepsis, oliguria and severity of illness [79]. The use of low-dose dopamine has been shown to be ineffective in halting the progression to acute renal failure in the critically ill [80, 81]. Daily intermittent haemodialysis is better than alternate-day haemofiltration in critically ill patients who require renal replacement therapy, improving the time to resolution and survival at 14 days [82]. Continuous renal replacement therapy has equivalent outcomes to intermittent renal replacement therapy for acute renal failure in critical illness, although the former may offer easier management of fluid balance in the haemodynamically unstable septic patient. Whether higher doses (i.e. ultrafiltration rates 35–45 vs. 20 ml kg−1 h−1) of continuous renal replacement therapy confer a survival advantage in acute renal failure awaits corroboration [83].
Metabolic management
Impaired adrenoceptor responsiveness has long been recognised in endotoxic shock, partially reversible by corticosteroids [84, 85]. However, high doses of steroids (methylprednisolone 30 mg/kg or dexamethasone), administered on day 1 of septic shock failed to show an outcome benefit in two multicentre randomised controlled trial in the 1980s, with the abandonment of empirical steroid treatment, except for those with demonstrable adrenocortical insufficiency [86, 87, 88]. However, later work employing the prospective characterisation of the adrenal status of patients in septic shock, through the use of a 250 μg ACTH stimulation test, into so-called responders (proposed unimpaired adrenocortical axis) and non-responders (proposed relative adrenocortical insufficiency) proved more encouraging. Thus non-responders randomised to 50 mg hydrocortisone every 6 h plus 50 μg oral fludrocortisone for 7 days displayed a significantly better 28-day vasopressor-withdrawal effect and survival advantage than those receiving placebo [89]. Overall survival between the hydrocortisone and placebo groups was not statistically different [90]. An ongoing trial (EUROCORTICUS) aims to address previous findings and investigate the risk-benefit ratio of low-dose steroids in non-refractory septic shock.
Glycaemic control, whilst avoiding potentially deleterious episodes of hypoglycaemia, plays an important role in outcomes of sepsis-associated organ failures and mortality. Tight glucose control (4.4–6.1 mmol/l) compared with standard care confers significant survival advantage in post-operative cardiac surgery patients. Multiple-organ failure with a proven focus of sepsis was also decreased [91]. Recent studies further support tight but less stringent control of blood glucose in critically ill patients (8.0 mmol/l or less) but suggest that glucose control, rather than insulin dose per se, is more important in determining outcome [92].
Anti-thrombotic strategies
The inflammatory response in severe sepsis is integrally related to procoagulant activity and endothelial activation. Protein C is activated by complexing with thrombin and endothelial cell thrombomodulin. Activated protein C (APC) then modulates inflammation, coagulation and endothelial cell function. A deficiency of APC and lower levels of protein C activity in sepsis are correlated with higher mortality rates [93, 94]. The PROWESS trial of drotrecogin alfa (activated) (recombinant human APC, rhAPC) showed that patients with severe sepsis who were randomised to 96-h infusions of rhAPC (24 μg kg−1 h−1) within 24 h of inclusion had significantly lower 28-day all-cause mortality vs. placebo (24.7% vs. 30.8% respectively). The incidence of serious bleeding was higher in the rhAPC group (3.5% vs. 2.0%, p = 0.06) [95], and it seems that sicker patients (APACHE II>25) benefit most from this therapy [96]. The effect was not reproduced in a large scale trial of anti-thrombin III in severe sepsis (mortality 38.9%, anti-thrombin group vs. 38.7% for placebo group) in spite of favourable indications from preclinical and phase II trials [97], in this sense mirroring experience with many other putative therapeutic interventions (i.e. anti-endotoxin, anti-TNF and nitric oxide synthase inhibition) trialled in patients with sepsis over many years [98, 99, 100, 101, 102, 103, 104, 105, 106, 107]. This failure (PROWESS notwithstanding) of new pharmacological therapies and immunotherapies in patients with sepsis may in part reflect the complexity of mechanisms leading to organ dysfunction and the consequent heterogeneity of the patient population. Whether new definitions are needed that may identify critically ill patients more likely to respond to novel therapies remains unclear [106].
Other strategies
Blood transfusion requirements in the critically ill have evolved from reports of its beneficial use dating back to 1935 and the appreciation of its value in improving tissue DO2 in early resuscitation [108]. However, the Transfusion in Critical Care Trial demonstrated that a conservative strategy employing a hemoglobin threshold of 7.0 g/dl (to maintain hemoglobin between 7 and 9 g/dl) is not associated with higher mortality than with a liberal transfusion protocol (i.e. threshold 10 g/dl), previously accepted as standard practice. However, only 6% of patients enrolled had sepsis, and in patients with ischaemic cardiac disease a higher threshold was recommended [109]. The optimal haemoglobin levels of specific groups of critically ill patients are therefore as yet unstudied, and the value of recombinant erythropoietin remains unclear.
Stress-ulcer prophylaxis to prevent clinically important bleeding from the gastrointestinal tract in critically ill patients is well established, and the predisposing factors (i.e. coagulopathy, hypotension and mechanical ventilation) are frequently present in patients with sepsis [110]. However, relatively small percentages of patients develop clinically important bleeding from recent observational studies. Moreover, the pursuit of early enteral nutrition where possible, together with a trend to an increased incidence of ventilator associated pneumonia by H2 antagonists/proton pump inhibitors, means that identifying subgroups of patients who may benefit most from stress ulcer prophylaxis remains difficult.
Conclusions
Multiple organ dysfunction complicating sepsis remains the commonest cause of mortality in the ICU. However, its mechanisms remain unknown, and the results of pathological autopsy studies show no correlation with degree of organ dysfunction or with specific causes of death. Nevertheless, these mechanisms continue to be unravelled, alongside emerging genetic predisposing targets. Moreover, the concept of a variable immune status, which can be tracked during sepsis and modulated, provides an increasing number of potential new therapeutic targets. A body of evidence accrued over decades reemphasises the fundamental importance of early recognition of physiological surrogates of tissue dysoxia in reducing associated organ dysfunction. Local and International clinical strategies, through a phased approach of the development of evidenced-based guidelines (incorporating proven strategies in sepsis), their implementation and evaluation, have undertaken the challenge of effecting improved survival in this patient population.
References
Members of the American College of Chest Physicians/Society of Crit Care Med Consensus Conference Committee: American College of Chest Physicians/Society of Crit Care Med Consensus conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 International Sepsis Definitions Conference. Crit Care Med 31:1250–1256
Brun-Buisson C (2000) The epidemiology of the systemic inflammatory response. Intensive Care Med 26 [Suppl 1]:S64–S74
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Annane D, Aegerter P, Jars-Guincestre MC, Guidet B (2003) Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 168:165–172
Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K (2003) Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 31:2332–2338
Eiseman B, Beart R, Norton L (1977) Multiple organ failure. Surg Gynecol Obstet 144:323–326
Fry DE, Pearlstein L, Fulton RL, Polk HC Jr (1980) Multiple system organ failure. Arch Surg 115:136–140
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Milberg JA, Davis DR, Steinberg KP, Hudson LD (1995) Improved survival of patients with acute respiratory distress syndrome 1983–1993. JAMA 273:306–309
Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR (2001) Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1389–1394
Fink MP, Evans TW (2002) Mechanisms of organ dysfunction in critical illness: report from a round table conference held in Brussels. Intensive Care Med 28:369–375
Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W (1985) A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J Clin Invest 76:1539–1553
Lam C, Tyml K, Martin C, Sibbald W (1994) Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 94:2077–2083
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Fink MP (2002) Bench-to-bedside review: cytopathic hypoxia. Crit Care 6:491–499
Sair M, Etherington PJ, Winlove CP, Evans TW (2001) Tissue oxygenation and perfusion in human skeletal muscle in patients with systemic sepsis. Crit Care Med 29:1343–1349
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223
Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riche F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JF (1999) Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA 282:561–568
Levi M, Ten Cate H (1999) Disseminated intravascular coagulation. N Engl J Med 341:586–592
Godin PJ, Buchman TG (1996) Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 24:1107–1116
Fink MP (2005) Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin 21:177–196
Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV (1986) Multiple-organ-failure syndrome: the gastrointestinal tract-the motor of MOF. Arch Surg 121:197–201
Fine J, Frank ED, Rutenberg SH, Schweinburg FB (1959) The bacterial factor in traumatic shock. N Engl J Med 260:214–220
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. A morphological, hemodynamic, and metabolic appraisal. Arch Surg 101:478–483
Fink MP, Antonsson JB, Wang HL, Rothschild HR (1991) Increased intestinal permeability in endotoxic pigs. Mesenteric hypoperfusion as an etiologic factor. Arch Surg 126:211–218
Matuschak GM, Rinaldo JE (1988) Organ interaction in the adult respiratory distress syndrome during sepsis: role of the liver in host defence. Chest 94:400–406
Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA (2000) Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 28:2591–2594
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Hotchkiss RS, Swanson PE, Freeman BD, et al (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27:1230–1251
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) Prognosis in acute organ-system failure. Ann Surg 202:685–693
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Lemeshow S, Klar J, Teres D, Avrunin JS, Gehlbach SH, Rapoport J, Rue M (1994) Mortality probability models for patients in the intensive care unit for 48 or 72 hours: a prospective, multicenter study. Crit Care Med 22:1351–1358
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Moreno R, Morais P (1997) Outcome prediction in intensive care: results of a prospective, multicentre Portuguese study. Intensive Care Med 23:177–186
Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H, Willatts S (1999) The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med 25:686–696
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Joly HR, Weil MH (1969) Temperature of the great toe as an indication of the severity of shock. Circulation 39:131–138
Yoshiya I, Shimada Y, Tanaka K (1980) Spectrophotometric monitoring of arterial oxygen saturation at the fingertip. Med Biol Eng Comput 18:27–32
Lundberg N, Troupp H, Lorin H (1965) Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. J Neurosurg 22:581–590
Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J (1992) Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 339:195–199
Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL (1996) Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 171:221–226
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Khalil HH, Richardson TQ, Guyton AC (1966) Measurement of cardiac output by thermal dilution and direct Fick method in dogs. J Appl Physiol 21:1131–1135
Branthwaite MA, Bradley RD (1968) Measurement of cardiac output by thermal dilution in man. J Appl Physiol 24:434–438
Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D (1970) Catheterisation of the heart in man with use of a flow directed balloon-tipped catheter. N Engl J Med 283:447–451
Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA (1996) The effectiveness of right heart catheterisation in the initial care of critically ill patients. SUPPORT investigators. JAMA 276:889–897
Matthay MA, Chatterjee K (1988) Bedside catheterisation of the pulmonary artery: risks compared with benefits. Ann Intern Med 109:826–834
Linton RA, Band DM, Haire KM (1993) A new method of measuring cardiac output in man using lithium dilution. Br J Anaesth 71:262–266
Orme RM, L'EPigott DW, Mihm FG (2004) Measurement of cardiac output by transpulmonary arterial thermodilution using a long radial artery catheter. A comparison with intermittent pulmonary artery thermodilution. Anaesthesia 59:590–594
Singer M, Clarke J, Bennett ED (1989) Continuous haemodynamic monitoring by esophageal Doppler. Crit Care Med 17:447–452
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Armstrong RF, Walker JS, Andrew DS, Cobbe SM, Cohen SL, Lincoln JC (1978) Continuous monitoring of mixed venous oxygen tension (PvO2) in cardiorespiratory disorders. Lancet I:632–634
Reinhart K, Kuhn HJ, Hartog C, Bredle DL (2004) Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med 30:1572–1578
Rady MY, Rivers EP, Nowak RM (1996) Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 14:218–25
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Weil MH, Shubin H, Biddle M (1964) Shock caused by Gram-negative organisms: analysis of 169 cases. Ann Intern Med 60:384–400
Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155
MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W (2004) Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 38:284–288
MacLean LD, Mulligan WG, McLean APH, Duff JH (1967) Patterns of septic shock in man—a detailed study of 56 patients. Ann Surg 166:543–562
Cochrane Injuries Group Albumin Reviewers (1998) Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 317:235–240
Choi PT, Yip G, Quinonez LG, Cook DJ (1999) Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med 27:200–210
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350:2247–2256
American Thoracic Society (2004) Evidence based colloid use in the critically ill. American Thoracic Society consensus statement. Am J Respir Crit Care Med 170:1247–1259
Shoemaker WC, Appel PL, Kram HB (1986) Hemodynamic and oxygen transport effects in critically ill general surgical patients. Crit Care Med 14:1032–1037
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high risk surgical patients. Chest 94:1176–1186
Boyd O, Grounds RM, Bennett ED (1993) A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high risk surgical patients. JAMA 270:2699–2707
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330:1717–1722
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med 333:1025–1032
Webb HH, Tierney DF (1974) Experimental pulmonary oedema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 110:556–565
Dreyfuss D, Soler P, Basset G, Saumon G (1988) High Inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137:1159–1164
Hickling KG, Henderson SJ, Jackson R (1990) Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16:372–377
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomised controlled trial. JAMA 280:159–165
Kramer P, Kaufhold G, Grone HJ, Wigger W, Rieger J, Matthaei D, Stokke T, Burchardi H, Scheler F (1980) Management of anuric intensive care patients with arteriovenous hemofiltration. Int J Artif Organs 3:225–230
Rasmussen HH, Ibels LS (1982) Acute renal failure; a multivariate analysis of causes and risk factors. Am J Med 73:211–218
Levy EM, Viscoli CM, Horowitz RI (1996) The effect of acute renal failure on mortality: a cohort analysis. JAMA 275:1489–1494
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ (1996) Acute renal failure in intensive are units-causes, outcome, and prognostic factors for hospital mortality. Crit Care Med 24:192–198
Goldberg LI, Mcdonald RH, Zimmerman AM (1963) Sodium diuresis produced by dopamine in patients with congestive heart failure. N Engl J Med 263:1060–1064
Australia and New Zealand Intensive Care Society (ANZICS) Clinical Trials group (2000) Low dose dopamaine in patients with early renal dysfunction: a placebo-controlled randomised trial. Lancet 356:2139–2143
Schiffl H, Lang SM, Fischer R (2002) Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346:305–310
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G (2000) Effects of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Weil MH, Shubin H, Biddle M (1964) Shock caused by Gram-negative micro-organisms: analysis of 169 cases. Ann Intern Med 60:384–400
Schumer W (1976) Steroids in the treatment of clinical septic shock. Ann Surg 184:333–341
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 317:653–658
Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, Duncan RC, Tendler MD, Karpf M (1984) The effects of high-dose corticosteroids in patients with septic shock. N Engl J Med 311:1137–1143
Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A (1998) Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26:645–650
Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E (2000) A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283:1038–1045
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367
Finney SJ, Zekveld C, Elia A, Evans TW (2003) Glucose control and mortality in critically ill patients. JAMA 2041–2047
Levi M, ten Cate B (1999) Disseminated intravascular coagulation. N Engl J Med 341:586–592
Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR (2001) Low levels of protein C are associated with poor outcomes in sepsis. Chest 120:915–922
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group (2001) Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Vincent JL (2003) Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS Trial. Crit Care Med 31:834–840
Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM; KyberSept Trial Study Group (2001) Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286:1869–1878
Ziegler EJ, McCutchan JA, Fierer J, Glauser MP, Sadoff JC, Douglas H, Braude AI (1982) Treatment of gram-negative bacteremia and shock with human anti-serum to a mutant Escherichia coli. N Engl J Med 307:1225–1230
Ziegler EJ, Fisher CJ Jr, Sprung CL, Straube RC, Sadoff JC, Foulke GE, Wortel CH, Fink MP, Dellinger RP, Teng NN; HA-1A Sepsis Study Group (1991) Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomised, double blind placebo controlled trial. N Engl J Med 324:429–436
Greenman RL, Schein RM, Martin MA, Wenzel RP, MacIntyre NR, Emmanuel G, Chmel H, Kohler RB, McCarthy M, Plouffe J; XOMA Sepsis Study Group (1991) A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. JAMA 266:1097–1102
Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA 273:934–941
Cohen J, Carlet J (1996) INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med 24:1431–1440
Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S (1998) Double blind randomised controlled trial of monoclonal antibody to human tumor necrosis factor in the treatment of septic shock. NORASEPT II Study Group. Lancet 351:929–933
Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C; Monoclonal Anti-TNF Randomized Controlled Sepsis Study Investigators (2004) Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 32:2173–2182
Marshall JC (2003) Much stuff as dreams are made on: mediator directed therapy in sepsis. Nat Rev Drug Discov 2:391–395
Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, Glauser M, Parsons P, Fisher CJ Jr, Repine JE (2000) Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med 28:232–235
Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R (2004) Multiple-center, randomised placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 32:21–30
Marriott HL, Kerwick A (1935) Continuous drip blood transfusion. Lancet 1:977–981
Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E; Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 340:409–17 (erratum: 340:1056)
Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, Wood G, Kirby A (1998) The Canadian Critical Care Trials Group. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med 338:791–797
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Evans, T.W. Organ dysfunction during sepsis. Intensive Care Med 32, 349–360 (2006). https://doi.org/10.1007/s00134-005-0038-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-0038-9