Abstract
Aims/hypothesis
Our aim was to compare the contributions of impaired beta cell function (IBF) and insulin resistance with the development of type 2 diabetes in a Japanese community.
Methods
A total of 2094 residents aged 40–79 years without diabetes underwent a health examination including a 75 g OGTT in 2007. Participants were divided into four groups according to the presence or absence of IBF (insulinogenic index/HOMA-IR ≤28.5) and insulin resistance (HOMA-IR ≥1.61) and were followed up for 7 years (2007–2014). Cox’s proportional hazards model was used to estimate HRs and 95% CIs for type 2 diabetes. The population attributable fractions (PAFs) due to IBF, insulin resistance, and their combination were calculated.
Results
At baseline, the prevalence of isolated IBF, isolated insulin resistance, and both IBF and insulin resistance were 5.4%, 24.1% and 9.5%, respectively. During the follow-up period, 272 participants developed type 2 diabetes. The multivariable-adjusted HRs (95% CI) and PAFs (95% CI) for type 2 diabetes were 6.3 (4.3, 9.2) and 13.3% (8.7, 17.7) in the participants with isolated IBF, 1.9 (1.3, 2.7) and 10.5% (4.0, 16.6) in those with isolated insulin resistance, and 8.0 (5.7, 11.4) and 29.3% (23.0, 35.1) in those with both IBF and insulin resistance, respectively, compared with the participants without either.
Conclusions/interpretation
The present study suggests that the combination of IBF and insulin resistance makes the main contribution to the development of type 2 diabetes in Japanese communities.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 2 diabetes mellitus compromises the quality of life of affected people by contributing to the development of micro- and macrovascular diseases, as well as cancers and dementia [1,2,3]. Insulin is the main peptide hormone that regulates glucose metabolism, and insulin resistance, which is mainly caused by obesity, is a pathological condition in which the insulin sensitivity is downregulated. Impaired insulin secretion and insulin resistance are thus two essential components in the pathophysiology of type 2 diabetes [4,5,6]. Several clinical studies reported that Asian individuals had a lower ability to secrete insulin than white individuals, whereas insulin resistance and obesity were more prevalent in white individuals [7,8,9]. In addition, it has been reported that the genetic predispositions linked with the ability to secrete insulin are associated with the development of diabetes among Asian populations [10,11,12,13,14]. Therefore, the magnitude of the contribution of the ability to secrete insulin and insulin resistance may vary among individuals of different ethnic and genetic backgrounds. Nevertheless, given the recent rising trend in the burden of obesity in Asia, it seems reasonable to speculate that the contribution of insulin resistance to the development of diabetes at the population level could be rising relative to that of impaired insulin secretion among Asian populations [9, 15].
On the other hand, insulin secretion is affected mutually by beta cell function and insulin resistance. Insulin secretion is accelerated compensatorily with the exacerbation of insulin resistance. A recent clinical study has reported that the beta cell functions estimated by the disposition index are comparable between Asian people and white people, and the lower insulin secretion ability in Asian people compared with white people can be explained by the higher insulin sensitivity due to the difference in body composition [8]. The disposition index, which is the ratio of measures of insulin secretion and insulin resistance, has been considered to be more appropriate than the index of insulin secretion to accurately assess beta cell function, because the disposition index accounts for the compensatory increase in insulin secretion associated with the exacerbation of insulin resistance [16].
However, there have been no population-based prospective cohort studies examining the population attributable risks of impaired beta cell function (IBF) as assessed by the disposition index and insulin resistance on the development of type 2 diabetes among general populations. The aim of the present study, therefore, was to compare the contributions of IBF and insulin resistance to the development of type 2 diabetes in a general Japanese population.
Methods
Study population
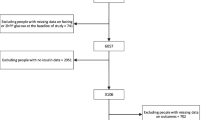
A population-based prospective cohort study investigating CVD, diabetes and lifestyle-related diseases has been underway since 1961 in the town of Hisayama, a suburb of the Fukuoka metropolitan area on Kyushu Island, Japan. According to the national census, the age and occupational distributions and major nutrient intake levels of the Hisayama population were similar to those of Japan during the period between 1960 and 2010 [17]. In 2007, a total of 2957 Hisayama residents aged 40–79 years (77.1% of the total population of this age group) underwent a health examination. After excluding eight individuals who did not consent to participate in the examination, 484 individuals with diabetes at baseline, 172 who did not undergo an OGTT, 58 whose data on insulinogenic index were not available at baseline, 14 whose data on alcohol consumption were missing at baseline, and 127 who did not undergo re-examinations during follow-up, the remaining 2094 individuals (880 men and 1214 women) were enrolled in the present study. This study was conducted with the approval of the Kyushu University Institutional Review Board for Clinical Research. Written informed consent was obtained from all participants.
Evaluation of glucose intolerance status, the status of beta cell function, and insulin resistance at baseline
At baseline, the study participants underwent a 75 g OGTT between 08:00 and 10:30 hours after an overnight fast of at least 12 h. Plasma glucose was obtained in a fasting state, and at 30 min and 2 h during an OGTT. Plasma glucose levels were determined by a hexokinase method. Glucose tolerance status was defined by the WHO criteria in 2006 as follows. Normal glucose tolerance: fasting plasma glucose (FPG) <6.1 mmol/l and 2 h postload glucose (2hPG) <7.8 mmol/l; prediabetes: FPG 6.1–6.9 mmol/l and 2hPG <7.8 mmol/l or FPG <7.0 mmol/l and 2hPG 7.8–11.0 mmol/l; and diabetes: FPG ≥7.0 mmol/l, 2hPG ≥11.1 mmol/l, and/or the use of glucose-lowering medications [18]. Serum insulin levels were determined by electrochemiluminescence immunoassay (ECLusys 2010; Roche Diagnostics, Basel, Switzerland). Insulin secretion was evaluated using the insulinogenic index, defined as [30 min insulin (pmol/l) − fasting insulin (pmol/l)] / [30 min glucose (mmol/l) − fasting glucose (mmol/l)] [19]. Insulin resistance was evaluated using the HOMA-IR, defined as fasting insulin (pmol/l) × fasting glucose (mmol/l) / 135 [20]. Disposition index was calculated as insulinogenic index/HOMA-IR [21]. IBF was defined as insulinogenic index/HOMA-IR ≤28.5, and insulin resistance was defined as HOMA-IR ≥1.61 based on the previous reports [22,23,24].
Participants were divided into 2 × 2 categories according to the presence or absence of IBF and insulin resistance: a normal group, a group with isolated IBF (individuals with IBF and without insulin resistance), a group with isolated insulin resistance (individuals with insulin resistance and without IBF), and a group with both IBF and insulin resistance.
For the sensitivity analyses, alternative indices for beta cell function and IR insulin resistance were calculated. The ratio of insulin AUC to glucose AUC during the OGTT (InsAUC/GluAUC) was calculated using the trapezoid method as ([fasting insulin (pmol/l) + 30 min insulin (pmol/l)] / 2 × 30 + [30-min insulin (pmol/l) + 2 h insulin (pmol/l) / 2 × 90]) / ([fasting glucose (mmol/l) + 30 min glucose (mmol/l)] / 2 × 30 + [30 min glucose (mmol/l) + 2 h glucose (mmol/l)] / 2 × 90) [25]. The Matsuda index was calculated as 10,000 per square root of {fasting glucose (mmol/l) × fasting insulin (pmol/l) × [fasting glucose (mmol/l) × 15 + 30 min glucose (mmol/l) × 60 + 2 h glucose (mmol/l) × 45] / 120 × [fasting insulin (pmol/l) × 15 + 30 min insulin (pmol/l) × 60 + 2 h glucose (pmol/l) × 45] / 120} according to the previously reported method [26, 27]. The product of InsAUC/GluAUC and the Matsuda index (InsAUC/GluAUC×Matsuda index) was used as an alternative index for beta cell function [21], and the Matsuda index was used as an alternative index for insulin resistance. The IBF and insulin resistance were defined alternatively as InsAUC/GluAUC×Matsuda index of ≤131.8 and Matsuda index of ≤4.97, respectively. The cut-off values of the insulinogenic index/HOMA-IR, InsAUC/GluAUC×Matsuda index, and Matsuda index were determined as the point maximising the Youden index [= max (sensitivity + specificity − 1)] that optimises the discriminatory ability for the risk of incident diabetes [28, 29] (ESM Table 1).
Follow-up survey and determination of type 2 diabetes
The participants were followed-up prospectively by an annual health examination including OGTT until 30 November 2014 (the median follow-up period was 6.9 years [range 0.6–7.4 years]; follow-up rate: 94.3%). During the follow-up period, the incidence of type 2 diabetes was defined by either the results of the OGTT or the measurements of plasma glucose as FPG ≥7.0 mmol/l, 2hPG or casual plasma glucose ≥11.1 mmol/l, and/or the use of glucose-lowering medications (oral hypoglycaemic agents, injectable glucagon-like peptide analogues, or insulin), according to the 2006 WHO criteria. When a participant died, we reviewed all available clinical information including the use of glucose-lowering medication. Participants were censored at the time of death, the latest occasion of the health examination, or the date of the diagnosis of type 1 diabetes (one participant was diagnosed as having type 1 diabetes during follow-up).
Measurement of other risk factors
Each participant completed a self-administered questionnaire covering: medical history; use of medications for hypertension, diabetes and dyslipidaemia; alcohol intake; smoking habits; and regular exercise during leisure time. Diabetes in first-degree relatives was considered as a present family history of diabetes. Alcohol intake and smoking habits were classified as either current use or not. Daily amounts of alcohol (g/day) were estimated according to the frequency of habitual alcohol intake per week or month, and the kinds and amounts of alcoholic beverages customarily consumed, and were classified into three categories: no (0 g/day), moderate (1–33 g/day) and heavy (over 34 g/day) alcohol intake. Participants engaging in sports or other forms of exertion at least three times per week during their leisure time were defined as the regular exercise group. BP was measured three times using a standard automated sphygmomanometer in the sitting position after at least 5 min of rest. The mean of these three measurements was used in the analysis. Hypertension was defined as BP ≥140/90 mmHg, and/or use of antihypertensive agents. The height and weight of each participant, wearing light clothes without shoes, were measured and BMI (kg/m2) was calculated. Obesity was defined as a BMI level ≥25.0 kg/m2. According to the obesity classifications of the Japan Society for the Study of Obesity, participants were also categorised into the following three groups: BMI <18.5, 18.5–24.9 and ≥25.0 kg/m2 [30]. Serum levels of total cholesterol, HDL-cholesterol and triacylglycerols were measured enzymatically.
Statistical analysis
Mean values and frequencies of potential risk factors for diabetes were estimated and compared between the normal group and the groups with IBF, insulin resistance, or both IBF and insulin resistance by using the generalised linear model, respectively. The insulinogenic index, HOMA-IR, insulinogenic index/HOMA-IR, and serum triacylglycerols were presented as median values and their IQRs. The incidence rates of type 2 diabetes were estimated using the person-years method. Cox’s proportional hazards model was used to estimate the HRs with 95% CIs for the incidence of diabetes in the groups with IBF, insulin resistance, or both IBF and insulin resistance. Adjustments were made for age, sex, BMI categories, family history of diabetes, serum total cholesterol, serum HDL-cholesterol, log-transformed serum triacylglycerols, use of lipid-lowering medication, hypertension, smoking habits, alcohol consumption and regular exercise. The interaction of IBF and insulin resistance was tested by using the relevant Cox model including the multiplicative interaction term. In the subgroup analyses, the participants were divided by obesity (BMI <25.0 or ≥25.0 kg/m2) and the status of glucose intolerance at baseline (normal glucose tolerance and prediabetes). The heterogeneities between subgroups were tested by adding a multiplicative interaction term of the status of IBF or insulin resistance with the indicator of the group to the relevant model. The population attributable fraction (PAF) for diabetes due to the status of IBF and insulin resistance at baseline was calculated using the formula PAF = PD (HR − 1) / HR, where PD denotes the proportion of total cases in the population arising from the cases exposed to a risk factor [31] and the multivariable-adjusted HR of each group compared with the normal group without either IBF or insulin resistance. The 95% CIs of the PAF were estimated by the method proposed by Greenland [32]. In addition, the risk of the development of type 2 diabetes according to the quartiles of indices of insulin secretion, insulin resistance and beta cell function were evaluated. Two-sided values of p < 0.05 were considered to indicate statistical significance in all analyses. Statistical analyses were conducted using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC, USA).
Results
The mean age of the total participants was 60.0 years, 42.0% were male, and the mean BMI was 23.0 kg/m2. The median value (IQR) of the insulinogenic index, HOMA-IR and insulinogenic index/HOMA-IR were 82.4 (49.3–147.1), 1.27 (0.88–1.88) and 63.4 (36.4–118.1), respectively. According to the baseline status of IBF and insulin resistance, there were 1277 normal participants (61.0%), 114 participants (5.4%) with isolated IBF, 504 (24.1%) with isolated insulin resistance, and 199 (9.5%) with both IBF and insulin resistance (Table 1). Participants with isolated IBF were the oldest and more likely to be male. They also had the highest frequencies of smoking habits and alcohol intake across the four categories. Participants with isolated insulin resistance had higher BMI and the highest frequencies of use of lipid-lowering medication. In this group, the insulinogenic index was highest, but the disposition index was lower than in the normal group. The participants with both IBF and insulin resistance had the highest median values of serum triacylglycerols and the highest frequency of family history of diabetes, prediabetes, obesity and hypertension. HOMA-IR was the highest in this group. In addition, they had the lowest disposition index, comparable to that in the participants with isolated IBF.
During the follow-up, 272 (13.0%) participants developed diabetes. The age- and sex-adjusted HRs of developing diabetes increased significantly in the groups with isolated IBF (HR 6.89, 95% CI 4.73, 10.05, p < 0.001), isolated insulin resistance (HR 2.11, 95% CI 1.51, 2.95, p < 0.001), and both IBF and insulin resistance (HR 9.87, 95% CI 7.27, 13.41, p < 0.001) compared with those with normal glucose tolerance (Table 2). This association remained unchanged after adjustment for age, sex, BMI categories, family history of diabetes, serum total cholesterol, serum HDL-cholesterol, log-transformed serum triacylglycerols, lipid-lowering medication, hypertension, smoking habits, alcohol consumption and regular exercise. Consequently, the contribution to the estimated proportion of cases was the highest in individuals with both IBF and insulin resistance (PAF 29.3%, 95% CI 23.0, 35.1%), followed in order by those with isolated IBF (PAF 13.3%, 95% CI 8.7, 17.7%) and those with isolated insulin resistance (PAF 10.5%, 95% CI 4.0, 16.6%) (Fig. 1).
PAFs for the development of type 2 diabetes according to baseline IBF and insulin resistance status in the overall population (a) or in subgroups stratified by BMI levels (b). The PAFs for diabetes were calculated using the proportion of total cases in the population arising from the cases exposed to a risk factor and the multivariable-adjusted HRs of each group compared with the group without IBF and insulin resistance, where the HRs were adjusted for age, sex, BMI (<18.5, 18.5–24.9, ≥25.0 kg/m2), family history of diabetes, hypertension, serum total cholesterol, serum HDL-cholesterol, serum triacylglycerols (log-transformed), use of lipid-lowering medication, current smoking, alcohol consumption and regular exercise in the overall analysis. BMI was excluded in the subgroup analysis of BMI. The status of IBF and insulin resistance were defined as IBF (−) and IR (−) for normal, IBF (+) and IR (−) for isolated IBF, IBF (−) and IR (+) for isolated IR, and IBF (+) and IR (+) for both IBF and IR, where IBF (+) and IR (+) were defined as disposition index (insulinogenic index/HOMA-IR) ≤28.5 and HOMA-IR ≥1.61, respectively. IR, insulin resistance
In both subgroups of BMI levels <25.0 and ≥25.0 kg/m2, the multivariable-adjusted HRs for the development of type 2 diabetes were the highest for the participants with both IBF and insulin resistance, followed in order by those with isolated IBF, those with isolated insulin resistance, and those with normal beta cell function and normal insulin resistance (Table 2). In the subgroup of BMI <25.0 kg/m2, the HRs (95% CIs) for the development of type 2 diabetes were 7.91 (5.10, 12.27) in participants with both IBF and insulin resistance; 6.53 (4.28, 9.96) in those with isolated IBF; and 1.84 (1.17, 2.89) in those with isolated insulin resistance. In the subgroup of BMI ≥25.0 kg/m2, the corresponding values were 9.28 (4.36, 19.77), 7.29 (2.78, 19.10) and 2.26 (1.03, 4.95), respectively. The PAFs of both IBF and insulin resistance and that of isolated IBF were equally high in the subgroup with BMI <25.0 kg/m2, while the PAF of both IBF and insulin resistance was highest, followed by that of isolated insulin resistance, in the subgroup with BMI ≥25.0 kg/m2 (Fig. 1).
Next, we estimated the difference in the contributions of IBF and insulin resistance status to the development of type 2 diabetes between subgroups of participants with normal glucose tolerance and prediabetes (Table 3). The contributions of isolated IBF, isolated insulin resistance, and both IBF and insulin resistance were similar in the participants with normal glucose tolerance, while in those with prediabetes, the PAF of both IBF and insulin resistance was the highest.
In the sensitivity analyses, the contributions of IBF and insulin resistance to the incidence of type 2 diabetes using an alternative index for beta cell function (InsAUC/GluAUC×Matsuda index) and insulin resistance (Matsuda index) (ESM Table 2) were similar to those using insulinogenic index/HOMA-IR and HOMA-IR (Tables 2 and 3). We also investigated the associations between the risk of type 2 diabetes and each of the following: the index of insulin secretion, insulin resistance and the disposition index. As a consequence, all indices were linearly associated with the development of type 2 diabetes (ESM Table 3).
Discussion
The present study demonstrated that the presence of IBF estimated by the disposition index, insulin resistance, or both IBF and insulin resistance were associated with increased risk of the development of type 2 diabetes, and approximately 30% of incident type 2 diabetes in a general Japanese population was attributable to the combination of IBF and insulin resistance, which made the strongest contribution to the development of type 2 diabetes. The PAF of isolated IBF and that of both IBF and insulin resistance for incident type 2 diabetes were similar at approximately 17% in participants without obesity, while approximately half of incident type 2 diabetes was attributable to both IBF and insulin resistance in those with obesity. In addition, the contributions of isolated IBF, isolated insulin resistance, and both IBF and insulin resistance on incident type 2 diabetes were similar in the participants with normal glucose tolerance, but the combination of IBF and insulin resistance made the strongest contribution in the prediabetic participants. Our findings suggest that the clinical importance of the management of insulin resistance, in addition to IBF, should receive more attention, in light of the recent obesity epidemic [17], for reducing the burden of type 2 diabetes in the Japanese population.
It has been reported that IBF has a great impact on the development of diabetes in Asian people [33], but this is still an area of controversy. A systematic review and meta-analysis of studies showed that Asian people have lower insulin secretion and lower insulin resistance than African people and white people [7]. Several cross-sectional studies suggested that impaired insulin secretion is the main cause of deteriorating glucose tolerance in Asian populations [34,35,36,37]. However, a recent clinical study reported that Asian people had lower insulin secretion and higher insulin sensitivity than white people, but the significant differences disappeared after adjusting for BMI, and the disposition index in both groups was comparable [8]. Insulin resistance upregulates insulin secretion, and insulin secretory capacity may appear normal in individuals with IBF [16]. On the other hand, even if beta cell function is normal, it may appear that there is impaired insulin secretion due to low insulin resistance. Therefore, it may be reasonable to suppose that beta cell function should be estimated by the disposition index, which considers both measures of insulin secretion and insulin resistance.
Several population-based studies have reported the magnitude of the contribution of impaired insulin secretion and insulin resistance [13, 14, 38]. Two prospective studies conducted in Asian populations, the Korean Genome and Epidemiology Study [13] and the Saku Study [14], reported that impaired insulin secretion made an approximately threefold greater contribution to the development of type 2 diabetes than insulin resistance. In contrast, the China Cardiometabolic Disease and Cancer Cohort (4C) Study of 95,000 people in China reported that the contribution of insulin resistance was greater than that of impaired insulin secretion [38]. In the present study, however, isolated IBF estimated by the disposition index and isolated insulin resistance made comparable contributions, and the contribution of both IBF and insulin resistance was pronouncedly higher. The exact reason for these discrepancies in findings is unclear, but it might be related to differences in the characteristics of participants. Individuals with normal glucose tolerance were enrolled in the Korean Genome and Epidemiology Study [13], and participants in the Saku Study may have been more health-conscious [14], since they participated in a hospital-based medical checkup of their own accord, while approximately 40% of participants in the 4C study were obese [38]. Moreover, the discrepancy may be in part attributable to the difference in the methods used to evaluate IBF, i.e. the index of insulin secretion in the previous studies vs the disposition index in the present study. Because the index of insulin secretion is affected by the extent of insulin resistance (e.g., the impact of impaired insulin secretion is likely to be underestimated in obese populations due to the compensatory secretion), the disposition index is thought to be more suitable for evaluating beta cell function to determine the contributions of impaired insulin secretion and insulin resistance. Our findings should be validated in a similar manner among other Asian populations.
The findings of the present study are important in terms of preventing the development of diabetes in Japanese individuals, in consideration of the pathophysiological changes in the general Japanese population. Our results revealed that the combination of IBF and insulin resistance made the greatest contribution to the process of incident diabetes, and that this effect was more prominent in obese individuals, possibly suggesting that the management of both insulin resistance and IBF has become increasingly important with the rising burden of obesity in Asian populations [9, 15]. The ADA guidelines recommend a diabetes prevention programme that emphasises weight reduction and moderate physical activity [39]. In clear agreement with this recommendation, several randomised controlled trials for Asians with impaired glucose tolerance have shown that lifestyle intervention including exercise and diet modifications significantly reduced the incidence of diabetes [40]. Intriguingly, the present study showed that IBF and both IBF and insulin resistance contributed to the same extent in the development of diabetes in non-obese individuals. Visceral fat, ectopic fat, muscle loss and a low-grade inflammation are generally considered to be involved in the development of type 2 diabetes through increased insulin resistance in non-obese individuals, especially in individuals with IBF [41,42,43,44]. Moreover, in the present study the PAFs of isolated IBF, isolated insulin resistance, and both IBF and insulin resistance for the onset of diabetes were similar in participants with normal glucose tolerance, while the PAF of the combination of IBF and insulin resistance was highest in participants with prediabetes. In this study, we could not assess whether IBF was due to genetic predisposition or decompensation for insulin resistance, but the Korean Genome and Epidemiology Study showed that insulin resistance worsened in the participants who developed diabetes during the follow-up period, and those with genetically low beta cell function could not compensate for worsened insulin resistance [13]. These findings suggest that insulin resistance plays as important a role in the mechanism of deteriorated glucose tolerance as IBF. Therefore, a public health approach to improving insulin resistance at an earlier stage of glucose intolerance is required to reduce the diabetic risk.
The strengths of the present study include its prospective population-based design, long-term duration of follow-up, high rate of participation and follow-up, and precise diagnosis of type 2 diabetes by including 75 g OGTT. Limitations of the present study should also be noted. The assessment of IBF and insulin resistance at baseline was based on a single measurement of plasma glucose and serum insulin concentration. During the follow-up, the levels of these risk factors could have been changed due to modifications of lifestyle, and thus misclassification of the status of IBF or insulin resistance was possible. This limitation might weaken the association observed in the current study, biasing the results toward the null hypothesis. Second, data on quantitative assessment of leisure physical activity were not available. Finally, the generalisability of our findings to other ethnic Asian populations may be limited, since we specifically considered glucose tolerance in a Japanese community. Studies in patients with different genetic and glucose tolerance backgrounds will be needed to confirm the applicability of our results to other populations.
In conclusion, the present study suggests that insulin resistance, in addition to IBF, contributed strongly to the development of type 2 diabetes in a Japanese population. It is reasonable to expect that the approach to improving insulin resistance will become increasingly crucial for reducing the burden of diabetes in future Asian populations.
Data availability
The datasets generated and analysed in the present study are not publicly available because they contain confidential clinical and demographic data of the study participants. However, further information about the datasets is available with permission of the principal investigator of the Hisayama Study (T. Ninomiya) on reasonable request for purposes of replicating procedures and results.
Abbreviations
- 2hPG:
-
2 h postload glucose
- FPG:
-
Fasting plasma glucose
- IBF:
-
Impaired beta cell function
- InsAUC/GluAUC:
-
Ratio of insulin AUC to glucose AUC during OGTT
- PAF:
-
Population attributable fraction
References
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258):405–412. https://doi.org/10.1136/bmj.321.7258.405
Carstensen B, Jorgensen ME, Friis S (2014) The epidemiology of diabetes and cancer. Curr Diab Rep 14(10):535. https://doi.org/10.1007/s11892-014-0535-8
Li W, Huang E (2016) An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis 53(2):393–402. https://doi.org/10.3233/JAD-160114
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104(6):787–794. https://doi.org/10.1172/JCI7231
Kasuga M (2006) Insulin resistance and pancreatic beta cell failure. J Clin Invest 116(7):1756–1760. https://doi.org/10.1172/JCI29189
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. https://doi.org/10.1038/nature05482
Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ (2013) Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 36(6):1789–1796. https://doi.org/10.2337/dc12-1235
Møller JB, Pedersen M, Tanaka H et al (2014) Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 37(3):796–804. https://doi.org/10.2337/dc13-0598
Yoon KH, Lee JH, Kim JW et al (2006) Epidemic obesity and type 2 diabetes in Asia. Lancet 368(9548):1681–1688. https://doi.org/10.1016/S0140-6736(06)69703-1
Fujimoto WY, Boyko EJ, Hayashi T et al (2012) Risk factors for type 2 diabetes: Lessons learned from Japanese Americans in Seattle. J Diabetes Investig 3(3):212–224. https://doi.org/10.1111/j.2040-1124.2012.00195.x
Cho YS, Chen CH, Hu C et al (2011) Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet 44(1):67–72. https://doi.org/10.1038/ng.1019
Suzuki K, Akiyama M, Ishigaki K et al (2019) Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet 51(3):379–386. https://doi.org/10.1038/s41588-018-0332-4
Ohn JH, Kwak SH, Cho YM et al (2016) 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol 4(1):27–34. https://doi.org/10.1016/S2213-8587(15)00336-8
Morimoto A, Tatsumi Y, Deura K et al (2013) Impact of impaired insulin secretion and insulin resistance on the incidence of type 2 diabetes mellitus in a Japanese population: the Saku study. Diabetologia 56(8):1671–1679. https://doi.org/10.1007/s00125-013-2932-y
Chan JC, Malik V, Jia W et al (2009) Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301(20):2129–2140. https://doi.org/10.1001/jama.2009.726
Bergman RN, Ader M, Huecking K, Van Citters G (2002) Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 51(Suppl 1):S212–S220. https://doi.org/10.2337/diabetes.51.2007.s212
Hata J, Ninomiya T, Hirakawa Y et al (2013) Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation 128(11):1198–1205. https://doi.org/10.1161/CIRCULATIONAHA.113.002424
World Health Organization & International Diabetes Federation (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF Consultation. Available from www.who.int/diabetes/publications/Definitionanddiagnosisofdiabetes_new.pdf. Accessed 1 December 2019
Phillips DI, Clark PM, Hales CN, Osmond C (1994) Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 11(3):286–292. https://doi.org/10.1111/j.1464-5491.1994.tb00273.x
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/bf00280883
Ahrén B, Pacini G (2004) Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 150(2):97–104. https://doi.org/10.1530/eje.0.1500097
The Japan Diabetes Society (2018) Treatment guide for diabetes 2018-2019. Bunkodo, Tokyo [guide in Japanese]
Kosaka K, Kuzuya T, Yoshinaga H, Hagura R (1996) A prospective study of health check examinees for the development of non-insulin-dependent diabetes mellitus: relationship of the incidence of diabetes with the initial insulinogenic index and degree of obesity. Diabet Med 13(9 Suppl 6):S120–S126
Nakai Y, Nakaishi S, Kishimoto H et al (2002) The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med 19(4):346–347. https://doi.org/10.1046/j.1464-5491.2002.00712_3.x
Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B (2008) Hyperbolic Relationship Between Insulin Secretion and Sensitivity on Oral Glucose Tolerance Test. Obesity 16(8):1901–1907. https://doi.org/10.1038/oby.2008.307
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22(9):1462–1470. https://doi.org/10.2337/diacare.22.9.1462
DeFronzo RA, Matsuda M (2010) Reduced time points to calculate the composite index. Diabetes Care 33(7):e93. https://doi.org/10.2337/dc10-0646
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845. https://doi.org/10.2307/2531595
Perkins NJ, Schisterman EF (2006) The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163(7):670–675. https://doi.org/10.1093/aje/kwj063
Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity (2002) New criteria for ‘obesity disease’ in Japan. Circ J 66(11):987–992. https://doi.org/10.1253/circj.66.987
Rockhill BNB, Weinberg C (1998) Use and misuse of population attributable fractions. Am J Public Health 88(1):15–19. https://doi.org/10.2105/ajph.88.1.15
Greenland S (1999) Re: “Confidence limits made easy: interval estimation using a substitution method”. Am J Epidemiol 149(9):884. https://doi.org/10.1093/oxfordjournals.aje.a009905
Yabe D, Seino Y, Fukushima M, Seino S (2015) β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 15(6):602. https://doi.org/10.1007/s11892-015-0602-9
Taniguchi A, Nakai Y, Fukushima M et al (1992) Pathogenic factors responsible for glucose intolerance in patients with NIDDM. Diabetes 41(12):1540–1546. https://doi.org/10.2337/diab.41.12.1540
Matsumoto K, Miyake S, Yano M et al (1997) Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care 20(10):1562–1568. https://doi.org/10.2337/diacare.20.10.1562
Kim DJ, Lee MS, Kim KW, Lee MK (2001) Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism 50(5):590–593. https://doi.org/10.1053/meta.2001.22558
Qian L, Xu L, Wang X et al (2009) Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev 25(2):144–149. https://doi.org/10.1002/dmrr.922
Wang T, Lu J, Shi L et al (2020) Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol 8(2):115–124. https://doi.org/10.1016/S2213-8587(19)30425-52
American Diabetes Association (2019) 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42(Suppl. 1):S29–S33. https://doi.org/10.2337/dc19-S003
Modesti PA, Galanti G, Calá P, Calabrese M (2016) Lifestyle interventions in preventing new type 2 diabetes in Asian populations. Intern Emerg Med 11(3):375–384. https://doi.org/10.1007/s11739-015-1325-2
Gujral UP, Weber MB, Staimez LR, Narayan KMV (2018) Diabetes Among Non-Overweight Individuals: an Emerging Public Health Challenge. Curr Diab Rep 18(8):60. https://doi.org/10.1007/s11892-018-1017-1
Cleasby ME, Jamieson PM, Atherton PJ (2016) Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 229(2):R67–R81. https://doi.org/10.1530/JOE-15-0533
Chuang HC, Tan TH (2017) MAP4K4 and IL-6+ Th17 cells play important roles in non-obese type 2 diabetes. J Biomed Sci 24(1):4. https://doi.org/10.1186/s12929-016-0307-7
Zhou J, Wang Y, He Y et al (2018) Non-obese type 2 diabetes patients present intestinal B cell dysregulations associated with hyperactive intestinal Tfh cells. Mol Immunol 97:27–32. https://doi.org/10.1016/j.molimm.2018.03.008
Acknowledgements
The authors thank the residents of the town of Hisayama for their participation in the survey and the staff of the Division of Health and Welfare of Hisayama for their cooperation with this study. The statistical analyses were carried out using the computer resources offered under the category of General Projects by the Research Institute for Information Technology, Kyushu University. Some of the data were presented as an abstract at the ADA 79th Scientific Sessions in 2019.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This study was supported in part by Grants-in-Aid for Scientific Research A (JP16H02692), B (JP17H04126, JP18H02737 and JP19H03863), C (JP18K07565, JP18K09412, JP19K07890, JP20K10503 and JP20K11020), Early-Career Scientists (JP18K17925) and Research Activity Start-up (JP19K23971) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (20FA1002); and by the Japan Agency for Medical Research and Development (JP20dk0207025, JP20km0405202 and JP20fk0108075).
Author information
Authors and Affiliations
Contributions
MY contributed to the study concept, data collection, data analysis, data interpretation and drafting of the manuscript. YH contributed to the study concept, data collection, interpretation of data and revision of the manuscript. JH, MH, TH, DY and NM contributed to the data collection and interpretation of data. UN and TK contributed to the interpretation of data and revision of the manuscript. TN was the chief investigator of the Hisayama Study and contributed to the study concept, data collection, interpretation of data and revision of the manuscript. All authors critically reviewed the manuscript and approved the final version. TN is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for its integrity and accurate analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM Tables
(PDF 291 kb)
Rights and permissions
About this article
Cite this article
Yoshinari, M., Hirakawa, Y., Hata, J. et al. Comparison of the contributions of impaired beta cell function and insulin resistance to the development of type 2 diabetes in a Japanese community: the Hisayama Study. Diabetologia 64, 1775–1784 (2021). https://doi.org/10.1007/s00125-021-05459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05459-7