Abstract
Aims/hypothesis
The Cre/loxP system, which enables tissue-specific manipulation of genes, is widely used in mice for diabetes research. Our aim was to develop a new Cre-driver mouse line for the specific and efficient manipulation of genes in pancreatic alpha cells.
Methods
A Gcg CreERT2 knockin mouse, which expresses a tamoxifen-inducible form of Cre from the endogenous preproglucagon (Gcg) gene locus, was generated by homologous recombination. The new Gcg CreERT2 mouse line was crossed to the Rosa26 tdTomato (R26 tdTomato) Cre reporter mouse line in order to evaluate the tissue specificity, efficiency and tamoxifen dependency of Gcg CreERT2-mediated recombination. Cell types of pancreatic islets were identified using immunohistochemistry. Biochemical and physiological data, including blood glucose levels, plasma glucagon and glucagon-like peptide (GLP)-1 levels, and pancreatic glucagon content, were collected and used to assess the overall effect of Gcg gene targeting on Gcg CreERT2/w heterozygous mice.
Results
Tamoxifen-treated Gcg CreERT2/w ;R26 tdTomato/w mice displayed Cre reporter activity, i.e. expression of tdTomato red fluorescent protein (RFP) in all known cells that produce proglucagon-derived peptides. In the adult pancreas, RFP was detected in 94–97% of alpha cells, whereas it was detected in a negligible (~ 0.2%) proportion of beta cells. While more than 98% of cells labelled with tamoxifen-induced RFP were glucagon-positive cells, 14–25% of pancreatic polypeptide (PP)-positive cells were also positive for RFP, indicating the presence of glucagon/PP bihormonal cell population. Tamoxifen-independent expression of RFP occurred in approximately 6% of alpha cells. In contrast to alpha cells and GLP-1-producing neurons, in which RFP expression persisted for at least 5 months after tamoxifen administration (presumably due to rare neogenesis in these cell types in adulthood), nearly half of RFP-positive intestinal L cells were replaced with RFP-negative L cells over the first 2 weeks after tamoxifen administration. Heterozygous Gcg CreERT2/w mice showed reduced Gcg mRNA levels in islets, but maintained normal levels of pancreatic and plasma glucagon. The mice did not exhibit any detectable baseline physiological abnormalities, at least in young adulthood.
Conclusions/interpretation
The newly developed Gcg CreERT2 knockin mouse shows faithful expression of CreERT2 in pancreatic alpha cells, intestinal L cells and GLP-1-producing neurons. This mouse line will be particularly useful for manipulating genes in alpha cells, due to highly specific and efficient CreERT2-mediated recombination in this cell type in the pancreas.
Similar content being viewed by others
Introduction
Pancreatic islet alpha cells are specialised to produce the glucagon that counteracts insulin for glucose homeostasis. Defects in insulin production and release by islet beta cells, insulin signalling to target organs or both are primary causes of diabetes mellitus. However, it has become evident that dysregulation of glucagon secretion by alpha cells also contributes to diabetes development and severity, indicating that alpha cells could also be a therapeutic target for better management of the disease [1, 2]. In addition, recent studies have suggested that alpha cells might be a potential source for the generation of new beta cells to cure diabetes [3,4,5].
The Gcg gene encodes preproglucagon, which consists of an N-terminal signal peptide and proglucagon. In the pancreas, the Gcg gene is only expressed in alpha cells. Outside the pancreas, the Gcg gene is expressed in intestinal L cells [6] and in a subset of neurons in the lower brain stem [7], most of which are in the nucleus of the solitary tract (NST) and some in the intermediate reticular nucleus [8, 9]. Tissue-specific differential processing of proglucagon yields glucagon in alpha cells, but yields glucagon-like peptide (GLP)-1 and GLP-2 in L cells and neurons. Intestinal GLP-1 is one of the incretins that are released after food intake and augment insulin secretion from beta cells, thereby lowering the blood glucose level [10]. GLP-1-producing NST neurons, so-called preproglucagon (PPG) neurons, project to multiple brain regions where GLP-1 receptors are expressed. This central GLP-1 controls neurological and cognitive functions, including appetite regulation and glucose homeostasis [11], and activation of PPG neurons reduces food intake and body weight in mice [9].
Mouse models have been extensively used in islet studies. The use of Cre/lox site-specific recombination systems, which allow cell-type-specific deletion or activation of genes by expressing Cre recombinase in distinct cell populations, has greatly enhanced our knowledge of islet biology, both in normal conditions as well as in the pathogenesis of diabetes. For genetic manipulation of alpha cells, the transgenic mouse line in which the Cre gene is expressed under the control of the 1.6 kb fragment of the rat Gcg gene promoter has been widely used over the years [12]. Previously, we have also generated Gcg-Cre transgenic mice using an 8 kb mouse Gcg promoter and codon-optimised Cre (improved Cre; iCre) [13], and other groups have developed Gcg-Cre mice using a construct based on the bacterial artificial chromosome (BAC) [9, 14]. The transgenic approach is unable to control the insertion site and copy number of transgenes, leading to some unpredictability. A relatively low recombination rate in alpha cells has been reported in some studies with the rat Gcg-Cre mouse [15, 16], perhaps due to silencing of the transgene [17], while we noted off-target recombination in a large percentage of beta cells in our mouse Gcg-Cre mouse despite the use of a large 8 kb promoter [13]. Although not conclusive, it has been suggested that the Gcg gene is expressed at low levels in beta cells or their progenitors, and amplified Gcg promoter activity due to multiple copies of transgene produced enough Cre to cause recombination, even though endogenous Gcg promoter activity was low. In fact, gene expression analysis of single mouse beta cells has consistently demonstrated that beta cells express genes for other islet hormones at very low levels [18, 19].
Given the need for more precise manipulations of alpha cells, we developed an alternative Cre-driver mouse line that enables specific and efficient Cre-mediated recombination in alpha cells. To this end, we designed a new Cre-driver mouse with the following features: (1) use of the Gcg promoter to drive Cre expression in alpha cells to take advantage of its strong and specific activity in alpha cells within the pancreas, even though there will also be activity in GLP-1-producing cells; (2) use of a knockin strategy to express Cre under the control of endogenous regulatory elements for Gcg gene transcription; and (3) use of CreERT2, a tamoxifen-inducible form of Cre, to reduce off-target recombination, which would more likely occur during embryogenesis and early postnatal development. Although we did not incorporate a bicistronic expression system into our knockin strategy due to concerns about CreERT2 expression levels, Ackermann et al recently developed Gcg IRES-CreERT2 knockin mice that express both glucagon and CreERT2 from a targeted Gcg allele and demonstrated specific and efficient Cre-mediated recombination in alpha cells [20]. Here, we describe our new Gcg CreERT2 knockin mouse, with additional information regarding a Gcg iCre knockin mouse that we also generated. Given the similarity of our Gcg CreERT2 knockin mice to those reported by Ackermann et al [20], the results from the extended characterisation of CreERT2 expression in our mice may be applicable to their mice, and thus would supplement their report.
Methods
Vector construct and gene targeting
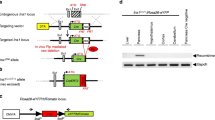
The BAC clone RP24-439G8, which harbours the Gcg gene locus of C57BL/6J mouse genomic DNA, was obtained from the BACPAC Resources Center at the Children’s Hospital Oakland Research Institute (Oakland, CA, USA) and directly modified to create a targeting vector using a galK-based BAC recombineering technique [21]. Briefly, a DNA sequence encoding the first 22 amino acids of preproglucagon in exon 2 was replaced with a CreERT2 coding sequence followed by an SV40 polyA (pA) signal. A neomycin resistance gene (neo) expression cassette flanked by flippase recognition target (FRT) sites was inserted immediately after the CreERT2-pA sequence. The modified exon 2, along with 2 kb upstream and 7 kb downstream sequences, was retrieved into the PGKdtabpA plasmid (a gift from P. Soriano, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Addgene plasmid no. 13440) [22] to add a diphtheria toxin A expression cassette to the end of 5′ homology arm (Fig. 1a). The resulting targeting construct was electroporated into F1 hybrid (129S6/SvEvTac × C57BL/6Ncr)-derived G4 embryonic stem (ES) cells [23] to obtain gene-targeted ES cells. Correctly targeted ES cells were screened by long-range PCR using LA Taq DNA polymerase (Takara Bio, Mountain View, CA, USA) and primers specific to the targeted allele and to the outside of targeting sequence (Fig. 1b). Chimeric mice were generated using a morula aggregation method.
Gene targeting for Gcg CreERT2 knockin mice. (a) Schematic representation of the Gcg locus, targeting vector and targeted alleles before and after FLPo-mediated removal of the neomycin resistance gene cassette (neo). Primers (P1–4), used for screening of targeted ES cells, are shown under the Gcg CreERT2-neo allele. Black boxes, Gcg coding regions; triangles, FRT sites. (b) PCR screening of targeted ES cells. Representative results of 5′ and 3′ PCR reactions in wild-type (−) or correctly targeted (+) ES cell clones are shown. M, size marker. (c) PCR genotyping of Gcg CreERT2-neo and Gcg CreERT2 mice using primers specific to CreERT2 and neomycin resistance gene
Plasmid and E. coli necessary for the BAC recombineering were a gift from Neal G. Copeland (National Cancer Institute, Frederick, MD, USA). CreERT2 fragment was obtained from pCAG-CreERT2 (a gift from C. Cepko, Harvard Medical School, Boston, MA, USA: Addgene plasmid no. 14797) [24].
Mice
Chimeric mice were bred with Rosa26 FLPo knockin mice (The Jackson Laboratory, Bar Harbor, ME, USA, stock no. 012930) to remove the neomycin resistance gene cassette (Fig. 1c), and resulting Gcg CreERT2/w mice were backcrossed to C57BL/6J mice (The Jackson Laboratory, stock no. 000664) for seven generations while collecting data for this study. The Rosa26 tdTomato Cre reporter (R26 tdTomato) strain was obtained from The Jackson Laboratory (stock no. 007909) and bred with Gcg CreERT2 mice to obtain double-mutant mice. Mouse genotypes were determined by PCR using DNA from tail biopsies. To induce Cre-mediated recombination, Gcg CreERT2 mice were treated with s.c. injection of tamoxifen dissolved in corn oil (10 mg/ml), using regimens indicated in the Results section.
Mouse colonies were maintained in a specific pathogen-free barrier facility in the Children’s Hospital of Pittsburgh of UPMC (Pittsburgh, PA, USA). All mouse handling and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh, and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Tissues were fixed by transcardiac perfusion of 4% paraformaldehyde in PBS for 10–15 min, followed by 6 h immersion in the same fixative. Overnight fixation was avoided to preserve fluorescence of dtTomato protein. Cryosections (6 μm) were used for all histological analyses. The primary antibodies used are listed in Table 1. These antibodies were validated using mouse tissues known to express and not to express the target protein. Secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA, USA). For staining with Cre antibody, an antigen retrieve was performed using a citrate-microwave method according to the manufacture’s protocol.
Blood glucose measurement and hormone assay
Blood glucose levels were measured using an Accu-Chek glucose meter (Roche, Indianapolis, IN, USA). Plasma glucagon and total GLP-1 levels were determined with glucagon RIA and a total GLP-1 ELISA kit (Millipore, Billerica, MA, USA), respectively. Pancreatic glucagon was measured as previously described [13]. All assays were performed in duplicate.
Islet isolation, cell sorting and gene expression analyses
For islet isolation, pancreas was digested by intraductal injection of CIzyme RI enzyme solution (VitaCyte, Indianapolis, IN, USA), followed by 15 min incubation at 37°C [25]. Islets were hand-picked after a density-gradient purification using Histopaque (Sigma-Aldrich, St Louis, MO, USA) [26] and dispersed into single cells in Accumax solution (Stemcell, Cambridge, MA, USA). Resulting single-cell populations were sorted on a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA) to isolate tdTomato red fluorescent protein (RFP)-positive and -negative cells. RNA was extracted from the isolated islets or the sorted cells using an RNeasy Mini kit (Qiagen, Hilden, Germany) or an Arcturus PicoPure RNA isolation kit (Applied Biosystems, Waltham, MA, USA), and cDNA was synthesised using a QuantiTect Reverse Transcription Kit (Qiagen). Gene expression was measured by real-time PCR using QuantiTect primers (Qiagen) and iTaq SYBR Green mix (Bio-Rad, Hercules, CA, USA). cDNA samples were run in duplicate.
Statistical analysis
Data are expressed as means ± SD. Statistical significance was assessed with an unpaired two-tailed Student t test or two-way ANOVA, and p < 0.05 was considered statistically significant. The experimenters were not blind to group assignment and no data were omitted.
Results
Alpha cell-specific expression of CreERT2 in Gcg CreERT2 mouse islets
We first examined CreERT2 expression in Gcg CreERT2/w mouse pancreas by immunohistochemistry using anti-Cre antibody. Since it is difficult to detect CreERT2 protein when the protein is distributed diffusely throughout the cytoplasm, Gcg CreERT2/w mice were treated with daily injection of 1 mg tamoxifen for 4 days at 2–3 weeks of age to accumulate CreERT2 proteins in the nucleus. Pancreases were fixed 1 day after the last tamoxifen injection and processed for immunohistochemistry. Cre staining was observed in glucagon-positive cells, but was hardly detected in insulin-positive cells in these mice (Fig. 2a). Because the gene targeting was designed to substitute CreERT2 expression for preproglucagon expression, homozygous Gcg CreERT2/CreERT2 mice were expected to be deficient in proglucagon-derived peptides. To confirm this, we generated Gcg CreERT2/CreERT2 mice and examined their islet morphology. Indeed, there was no glucagon staining observed. In addition, Gcg CreERT2/CreERT2 mouse islets displayed the expected hyperplasia of Cre-positive cells that were identified as alpha cells by positive staining for MafB (Fig. 2b). Alpha cell hyperplasia is the typical phenomenon in glucagon or glucagon receptor deficiency. Thus, we verified that the Gcg CreERT2 allele functions as expected.
Cre expression in Gcg CreERT2 mice. (a) Triple immunostaining for Cre, insulin and glucagon on Gcg w/w and Gcg CreERT2/w mouse pancreatic sections. Cre staining is merged with glucagon staining or insulin staining. DAPI nuclear staining is shown in blue. Scale bars, 50 μm. (b) Immunostaining for Cre and MafB on Gcg CreERT2/CreERT2 mouse pancreatic sections. Merged images with co-stained insulin (green) and DAPI (blue) are shown to the right. Scale bars, 50 μm
Tamoxifen-induced recombination in Gcg CreERT2 mouse alpha cells
To assess tissue specificity and efficiency as well as the tamoxifen dependency of recombination mediated by CreERT2 in Gcg CreERT2 mice, we bred Gcg CreERT2 mice with R26 tdTomato Cre reporter mice. The R26 tdTomato mouse expresses tdTomato RFP when Cre excises the transcription stop cassette. Because of robust expression of the tdTomato gene through the CAG promoter and exceptional brightness of tdTomato fluorescence, RFP-positive cells were easily identified on sections subjected to immunostaining. To examine CreERT2 activity in embryonic stages, pregnant female mice were injected with 1 mg tamoxifen at 11.5 days postcoitum for embryonic day (E)13.5 pancreases or at 14.5 days postcoitum for E16.5 pancreases. Double staining for glucagon and insulin on Gcg CreERT2/w ;R26 tdTomato/w pancreatic sections revealed that more than 60% of glucagon-positive cells expressed RFP on E13.5 (Fig. 3a), and that RFP expression expanded to more than 80% of glucagon-positive cells on E16.5 (Table 2). At E13.5, about 10% of insulin-positive cells were RFP-positive, and most were also positive for glucagon staining. It is known that co-expression of glucagon and insulin occurs in pancreatic endocrine cells at early developmental stages, but not in late stages [12, 27]. Consistently, we observed that fewer than 1% of insulin-positive cells were labelled with RFP in E16.5 pancreases (Table 2).
CreERT2-mediated recombination in Gcg CreERT2/w ;R26 tdTomato/w mice. (a) Detection of tdTomato epifluorescence (red) and co-immunostaining for glucagon (green) and insulin (blue) in Gcg CreERT2/w ;R26 tdTomato/w embryonic pancreas at E13.5. Scale bars, 50 μm. (b) Detection of tdTomato epifluorescence (red) and immunostaining for glucagon (green) on 2-month-old (2M) and 5-month-old (5M) non-treated (−) or tamoxifen (Tam)-treated (+) Gcg CreERT2/w ;R26 tdTomato/w mouse pancreatic sections. DAPI nuclear staining is shown in blue. Scale bars, 50 μm. (c) Detection of tdTomato epifluorescence (red) and co-immunostaining for glucagon (green) and PP (blue) on a 5-month-old tamoxifen-treated Gcg CreERT2/w ;R26 tdTomato/w mouse pancreatic section. Arrows indicate an RFP-positive cell stained with both glucagon and PP antibodies. Scale bars, 20 μm. (d) Representative flow plot showing clear separation of tdTomato RFP-positive cells (red) from RFP-negative cells (purple) by FACS. (e, f) qRT-PCR analysis of gene expression in tdTomato RFP-positive and -negative cell populations. Relative expression levels of Gcg gene (e) and Ins2 gene (f) to Ppia gene are shown. n = 3. Data are means ± SD
We next examined CreERT2-mediated recombination in adult mice. At 5 weeks of age, Gcg CreERT2/w ;R26 tdTomato/w mice were randomised into groups receiving either three doses of 1 mg tamoxifen or vehicle over a week, and pancreases were fixed at 2 or 5–6 months of age. Pancreatic sections were double-stained for glucagon and insulin or for pancreatic polypeptide (PP) and somatostatin. Although some RFP-positive cells were seen in the islets of vehicle-injected mice at both ages examined, a dramatic increase in RFP expression was observed among alpha cells in tamoxifen-injected mice at 2 months of age, and the pattern of RFP expression was maintained to 5–6 months of age (Fig. 3b). Quantitative analysis further demonstrated an efficient induction of RFP expression by tamoxifen in alpha cells (> 90%), with low frequency of tamoxifen-independent (‘leaky’) RFP expression (< 6%) in this cell type (Table 2). Importantly, more than 98% of RFP-positive cells were alpha cells, indicating that CreERT2-mediated recombination occurred almost exclusively in alpha cells. Consistently, RFP expression in beta and delta cells was negligible. However, a substantial proportion of PP cells (10–25%) were labelled with RFP (Table 2). Since only a few RFP-positive cells were negative for glucagon staining (1.2 ± 0.7% and 1.1 ± 0.5% of total RFP-positive cells at 2 and 5–6 months of age, respectively), this indicates that some alpha cells stained positively with anti-PP antibody. Indeed, double staining for glucagon and PP demonstrated the co-existence of both hormones in some cells (Fig. 3c), which is consistent with previous immunohistochemical studies on rat [28] or mouse [19] islets.
In addition to morphological assessments, expression of Gcg and Ins2 genes was measured by quantitative RT-PCR (qRT-PCR) in flow-sorted RFP-positive and -negative islet cells (Fig. 3d). Gcg gene expression was detected nearly exclusively in RFP-positive cells (Fig. 3e), whereas Ins2 gene expression was detected solely in RFP-negative cells, as expected (Fig. 3f). Collectively, the data confirmed that recombination of the floxed target sequence is tamoxifen-dependent and highly specific to alpha cells in Gcg CreERT2 mouse pancreases.
Recombination by Gcg CreERT2 in extrapancreatic proglucagon-producing cells
Besides pancreatic alpha cells, the Gcg gene is expressed in intestinal L cells and some neurons in the brain stem in which proglucagon is processed to GLP-1. In contrast to pancreatic alpha cells, the population of which is normally maintained by replication of existing alpha cells, L cells are constantly replenished by neogenesis from intestinal progenitor cells, and thus the population of labelled cells by an inducible Cre is assumed to diminish as time passes after tamoxifen exposure. For this reason, we examined intestine obtained from 3- to 4-month-old Gcg CreERT2/w ;R26 tdTomato/w mice that were treated with tamoxifen at 2–5 days, 2 weeks or 3 months before harvesting tissue. RFP-positive cells were occasionally observed in the epithelium of small intestine 2–5 days after tamoxifen treatment (Fig. 4a), and these cells stained positively with anti-GLP-1 antibody, indicating that CreERT2-mediated recombination occurred in L cells (Fig. 4b). Quantitative analysis revealed that 80.7 ± 3.2% of GLP-1-positive cells were RFP positive at 2–5 days after tamoxifen treatment. The proportion of RFP-expressing cells in the GLP-1-positive cell population decreased to 42.9 ± 12.2% and to only 2.6 ± 2.4% at 2 weeks and 3 months after tamoxifen administration, respectively. The analysis also showed that 11.1 ± 8.8% and 21.1 ± 7.8% of RFP-positive cells were negative for GLP-1 staining at 2–5 days and 2 weeks after tamoxifen treatment, respectively. It remains to be determined if these cells were L cells that had ceased GLP-1 production, or were perhaps other types of enteroendocrine cells.
CreERT2-mediated recombination in 2-month-old Gcg CreERT2/w ;R26 tdTomato/w mouse intestine and brain. (a) Jejunum section showing tdTomato epifluorescence (red) and immunostaining for E-cadherin (green). DAPI nuclear staining is shown in blue. Scale bar, 50 μm. (b) Jejunum section showing tdTomato epifluorescence (red) and co-immunostaining for GLP-1 (green) and E-cadherin (blue). Scale bar, 50 μm. (c) Brain section showing tdTomato-positive neurons in the NST. The area in the small square is shown at high magnification in the inset. Scale bar, 200 μm
In the brain, RFP expression was detected in neurons of the NST, but not in other areas (Fig. 4c). As expected, RFP-positive neurons were still seen 5 months after tamoxifen injection.
Normal physiology in Gcg CreERT2/w mice
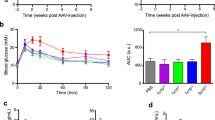
An effect of haploinsufficiency on phenotype has not been reported in global Gcg null mutant mice. To confirm that our Gcg CreERT2 mice also do not have any phenotypical changes due to heterozygosity, we collected biochemical and physiological data from wild-type Gcg w/w and Gcg CreERT2/w mice at 2–3 months of age. Measurements of gene expression in islets by qRT-PCR demonstrated a 60% reduction in Gcg mRNA levels but no change in Ins2 mRNA levels in Gcg CreERT2/w mice compared with wild-type mice (Fig. 5a, b). However, pancreatic glucagon content and plasma glucagon levels were normal in Gcg CreERT2/w mice, indicating that glucagon net content is maintained at normal levels by post-transcriptional mechanisms in the presence of Gcg gene heterozygosity (Fig. 5c, d). In addition, plasma total GLP-1 levels were not affected by disruption of the Gcg gene in the Gcg CreERT2 allele (Fig. 5e). Consistently, there were no differences in body weight or blood glucose levels in either the fed or fasted conditions between Gcg w/w and Gcg CreERT2/w mice (Fig. 5f, g).
Biochemical and physiological variables in 2- to 3-month-old Gcg CreERT2/w mice. (a, b) qRT-PCR analysis of gene expression in isolated islets. Relative expression levels of Gcg gene (a) and Ins2 gene (b) to Ppia gene are shown. n = 3. *p < 0.05, unpaired two-tailed Student t test. (c) Pancreatic glucagon content. (d) Plasma glucagon levels. (e) Plasma total GLP-1 levels. (f) Body weight. (g) Blood glucose levels. White circles, Gcg w/w; black squares, Gcg CreERT2/w. Data represent individual values and means ± SD
Gcg iCre knockin mice with an unexpected duplication mutation of the Gcg locus
We generated Gcg iCre knockin mice using the same strategy as the Gcg CreER2 mice, with the only difference being insertion of an iCre coding sequence instead of a CreERT2 sequence. While the iCre sequence was knocked into the Gcg gene as we designed, subsequent analysis of Gcg iCre/CreERT2 hemizygous mice, in which we observed glucagon expression, indicated that the targeted allele still carried an intact Gcg gene, and that a duplication mutation had occurred in the Gcg iCre mouse line, most likely during homologous recombination for gene targeting in ES cells. To determine the structure of the duplication, we performed a long-range PCR analysis of Gcg iCre/CreERT2 mouse genomic DNA using primers specific to iCre or the 3′ portion of exon 2, which does not exist in the Gcg iCre or Gcg CreERT gene, in combination with various primers for upstream and downstream of the Gcg gene. The results revealed that the duplicated region included all exons of the Gcg gene, which spans 9 kb, and extended upstream for at least 17 kb and downstream for at least 7.8 kb. The 3′ portion of the adjacent Fap gene, which is located upstream of Gcg and encodes fibroblast activation protein, was involved in the duplication. Due to such a large size of the duplication, we could not determine duplication junction points or the relative position of the Gcg iCre gene to the Gcg gene. Despite the lack of full information, we thought it was worthwhile to characterise this mouse line because the Gcg iCre gene has at least 17 kb upstream sequence of the Gcg gene.
Immunohistochemical examination of Gcg iCre/w ;R26 TdTomato/w mice at 3 weeks and 2 months of age demonstrated that most alpha cells were labelled with RFP (Fig. 6a, Table 3). While quantitative analysis showed no difference in the labelling efficiency in alpha cells between Gcg iCre/w and tamoxifen-treated Gcg CreERT2/w mice, slight but significant increases in RFP expression were observed in beta and PP cells in Gcg iCre/w mice compared with Gcg CreERT2/w mice (p < 0.0001 in beta cells and p = 0.0006 in PP cells, two-way ANOVA). Consistently, 3–6% of RFP-positive cells were negative for glucagon staining in Gcg iCre/w mouse pancreases.
Cre-mediated recombination in 2-month-old Gcg iCre/w ;R26 tdTomato/w mice. (a) Detection of tdTomato epifluorescence (red) and co-immunostaining for glucagon (green) and insulin (blue) on a pancreatic section. Scale bar, 50 μm. (b) Jejunum section showing tdTomato epifluorescence (red) and co-immunostaining for GLP-1 (green) and E-cadherin (blue). Cells positive for RFP and GLP-1 (arrowheads) and positive for GLP-1 but not for RFP (arrows) are seen. Scale bar, 50 μm. (c, d) Detection of tdTomato epifluorescence (red) and staining for myelin (green) on brain sections, including the region of the hypothalamus (c) and the brain stem (d)
In the intestine, RFP expression was observed in L cells that were marked with GLP-1 staining (Fig. 6b). RFP-negative L cells were often seen in crypts, indicating a time lag between GLP-1 expression and RFP expression in newly formed L cells, with the latter requiring iCre expression and recombination of the R26 TdTomato gene prior to RFP expression. In contrast to Gcg CreERT2 mice, non-specific RFP expression was widely seen in the brain in Gcg iCre/w ;R26 TdTomato/w mice (Fig. 6c, d). RFP-labelled cells often formed large clusters, suggesting that the recombination of the R26 TdTomato gene occurred in early developmental stages with subsequent clonal expansion (Fig. 6d).
Discussion
While we and Ackermann et al [20] used a knockin strategy to generate new Gcg CreERT2 or Gcg iCre mouse lines, two other groups have established new Gcg-Cre mouse lines by means of BAC transgenesis and successfully applied them to gene manipulation in L cells [14] or PGP neurons [9]. BAC transgenes include long flanking sequences on both sides of the Cre inserted into the Gcg gene, and thereby confer better control of Gcg-Cre gene expression by cis elements similar to the endogenous Gcg gene. Nevertheless, it was reported that approximately 20% of beta cells were labelled with Cre reporter in one of the BAC transgenic lines [29]. This is in contrast to our two new mouse models, in which activation of Cre reporter in beta cells was negligible (< 1%), even in the Gcg iCre mice in which the targeted Gcg gene locus was disarranged by a duplication mutation.
Upon tamoxifen injection, the Gcg CreERT2 mice displayed Cre reporter activation specifically in cells that express preproglucagon in adult mice. However, labelling NST neurons by the reporter seemed less effective compared with a recent study using BAC Gcg-Cre mice [9]. A further study is required to determine the efficiency of Cre-mediated recombination in the Gcg CreERT2 mouse brain, but it is possible that induction of recombination by tamoxifen is weaker in the brain than in other organs since the penetration of 4-hydroxytamoxifen, the primary active tamoxifen metabolite, into the brain is partially restricted by the P-glycoprotein (ABCB1) transporter [30]. In contrast, the efficiency of Cre reporter activation in Gcg CreERT2 mouse L cells was similar to that in the BAC Gcg-iCre mice [14]. Consistent with previous estimates of the turnover time of enteroendocrine cells using 3H-thymidine labelling [31], half of the Cre reporter-positive L cells disappeared during the first 2 weeks after tamoxifen treatment, indicating that the effect of genetic manipulation on L cells does not last longer in this inducible system. Taken together, our Gcg CreERT2 mouse favours studies on alpha cells, although each study must give careful consideration to the possible effects of Cre-mediated gene modifications in extrapancreatic sites.
The Gcg IRES-CreERT2 mice reported by Ackermann et al express both preproglucagon and CreERT2 as two independent proteins from a single Gcg-IRES-CreERT2 mRNA [20]. The Gcg iCre mice described here express preproglucagon in addition to Cre from the targeted allele due to a duplication mutation. In contrast, our Gcg CreERT2 mice are heterozygous null mutants for the Gcg gene. Although we did not detect any physiological abnormalities in the current analysis, Gcg heterozygosity may affect the functionality of preproglucagon-producing cells in extreme conditions or during ageing. Therefore, including Gcg CreERT2 mice as a control is crucial.
The lineage labelling analysis of the Gcg CreERT2 and Gcg iCre mouse islets showed that 14–25% of PP-positive cells co-expressed glucagon in adult animals. This result is comparable with findings from a recent study that identified glucagon/PP double-positive cells (16% of PP-positive cells) by RNA fluorescence in situ hybridisation analysis of mouse islet cells [19]. Our data showed that the size of this bihormonal cell population was relatively stable over the experimental period, suggesting the presence of a subpopulation of alpha cells. Lineage analysis using PP cell-specific Cre might determine if this subpopulation is an independent lineage from the majority of alpha cells that are negative for PP.
In conclusion, we describe a basic characterisation of a new Gcg CreERT2 knockin mouse. The data demonstrate that this mouse line is a useful tool, particularly for studies on pancreatic alpha cells. In addition to Gcg CreERT2 knockin mice, we report a new Gcg iCre knockin mouse that also displayed specific and efficient recombination in alpha cells. However, it should be noted that the Gcg iCre knockin mouse line is not adequate for some studies due to widespread Cre lineage labelling in the brain.
The mouse strains reported here are available at The Jackson Laboratory as stock no. 030681 for Gcg CreERT2 mice and no. 030663 for Gcg iCre mice.
Abbreviations
- BAC:
-
Bacterial artificial chromosome
- E:
-
Embryonic day
- ES:
-
Embryonic stem
- FRT:
-
Flippase recognition target
- GLP:
-
Glucagon-like peptide
- iCre:
-
Improved Cre
- NST:
-
Nucleus of the solitary tract
- PP:
-
Pancreatic polypeptide
- PPG neuron:
-
Preproglucagon neuron
- qRT-PCR:
-
Quantitative RT-PCR
- RFP:
-
Red fluorescent protein
References
D’Alessio D (2011) The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab 13(Suppl 1):126–132
Unger RH, Cherrington AD (2012) Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122:4–12
Thorel F, Nepote V, Avril I et al (2010) Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464:1149–1154
Ben-Othman N, Vieira A, Courtney M et al (2017) Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168:73–85
Chakravarthy H, Gu X, Enge M et al (2017) Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab 25:622–634
Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM (2008) Glucose sensing in L cells: a primary cell study. Cell Metab 8:532–539
Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C (1997) Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77:257–270
Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S (2011) Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 180:111–121
Gaykema RP, Newmyer BA, Ottolini M et al (2017) Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 127:1031–1045
Drucker DJ (2006) The biology of incretin hormones. Cell Metab 3:153–165
Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ (2017) Glucagon-like peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol Metab 28:88–103
Herrera PL (2000) Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127:2317–2322
Shiota C, Prasadan K, Guo P et al (2013) α-Cells are dispensable in postnatal morphogenesis and maturation of mouse pancreatic islets. Am J Physiol Endocrinol Metab 305:E1030–E1040
Parker HE, Adriaenssens A, Rogers G et al (2012) Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55:2445–2455
Solomou A, Meur G, Bellomo E et al (2015) The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic α-cells for hypoglycemia-induced glucagon secretion. J Biol Chem 290:21432–21442
Tuduri E, Denroche HC, Kara JA, Asadi A, Fox JK, Kieffer TJ (2014) Partial ablation of leptin signaling in mouse pancreatic α-cells does not alter either glucose or lipid homeostasis. Am J Physiol Endocrinol Metab 306:E748–E755
Magnuson MA, Osipovich AB (2013) Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab 18:9–20
Katsuta H, Akashi T, Katsuta R et al (2010) Single pancreatic beta cells co-express multiple islet hormone genes in mice. Diabetologia 53:128–138
Xin Y, Kim J, Ni M et al (2016) Use of the Fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proc Natl Acad Sci U S A 113:3293–3298
Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH (2017) High-fidelity glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol Metab 6:236–244
Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33:e36
Soriano P (1997) The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124:2691–2700
George SH, Gertsenstein M, Vintersten K et al (2007) Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci U S A 104:4455–4460
Matsuda T, Cepko CL (2007) Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A 104:1027–1032
Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG (2012) Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp 67:4137
McCall MD, Maciver AH, Pawlick R, Edgar R, Shapiro AM (2011) Histopaque provides optimal mouse islet purification kinetics: comparison study with Ficoll, iodixanol and dextran. Islets 3:144–149
Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D (1993) Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 118:1031–1039
Huang YH, Sun MJ, Jiang M, BY F (2009) Immunohistochemical localization of glucagon and pancreatic polypeptide on rat endocrine pancreas: coexistence in rat islet cells. Eur J Histochem 53:81–85
Soedling H, Hodson DJ, Adrianssens AE et al (2015) Limited impact on glucose homeostasis of leptin receptor deletion from insulin- or proglucagon-expressing cells. Mol Metab 4:619–630
Iusuf D, Teunissen SF, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH (2011) P-glycoprotein (ABCB1) transports the primary active tamoxifen metabolites endoxifen and 4-hydroxytamoxifen and restricts their brain penetration. J Pharmacol Exp Ther 337:710–717
Tsubouchi S, Leblond CP (1979) Migration and turnover of entero-endocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H-thymidine into mice. Am J Anat 156:431–451
Acknowledgements
We thank A. J. Styche (Children’s Hospital of Pittsburgh) for technical assistance in performing flow cytometry.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Data availability
The data are available on request from the corresponding authors.
Funding
This work was supported in part by the Children’s Hospital of Pittsburgh Foundation to CS and by National Institutes of Health grants to GKG (DK098196, DK111460, DK112836).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
CS, XX and GKG contributed to the conception and design of the research. CS, KP, PG and JF performed the experiments. CS prepared the figures and drafted the manuscript. GKG edited the manuscript. All authors contributed to the interpretation of the data and to the critical revision of the manuscript, and approved the final manuscript. CS and GKG are responsible for the integrity of the work as a whole.
Rights and permissions
About this article
Cite this article
Shiota, C., Prasadan, K., Guo, P. et al. Gcg CreERT2 knockin mice as a tool for genetic manipulation in pancreatic alpha cells. Diabetologia 60, 2399–2408 (2017). https://doi.org/10.1007/s00125-017-4425-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4425-x