Abstract
Aims/hypothesis
A new differentiation pathway for CD4−CD8− (DN) T cells has recently been identified that exhibits the potent function of peripheral converted DN T cells in suppressing immune responses and provides the potential to treat autoimmune diseases. The aim of this study was to determine if the DN T cells converted from CD4+ T cells of NOD mice retain the antigen-specific regulatory capacity and prevent autoimmune diabetes in vivo. We also sought to determine if the combination of DN T cells with rapamycin promotes islet allograft survival in autoimmune diabetic NOD recipients.
Methods
NOD CD4+ T cells were converted to DN T cells in an in vitro mixed-lymphocyte reaction, with or without GAD65 peptide, as previously reported. The antigen-specific DN T cells were adoptively transferred to NOD/SCID mice, new-onset diabetic NOD mice or islet-allograft-recipient NOD mice as the part of cell-based therapy. The development of diabetes and allograft survival was assessed by monitoring blood glucose levels.
Results
NOD CD4+ T cells were converted in vitro to DN T cells at a rate of 50% and expressed unique cell features. The DN T cells from NOD donors blocked autoimmunity and prevented diabetes in NOD models, and these effects were even greater for GAD65-peptide-primed DN T cells. DN T cells acted in conjunction with rapamycin to suppress alloantigen-triggered T cell proliferation, promoted apoptosis and prolonged islet allograft survival in NOD recipients.
Conclusions/interpretation
Administration of the islet beta cell antigen-specific DN T cells can prevent the development of autoimmune diabetes and promote islet allograft survival in NOD mice.
Similar content being viewed by others
Introduction
Type 1 diabetes is an autoimmune disease in which the host loses tolerance to self-antigens at a young age. Consequently, auto-aggressive T cells infiltrate the pancreas and, over time, selectively destroy the insulin-producing beta cells, leading to overt diabetes [1]. Although there are a variety of mechanisms involved, convincing evidence indicates that the regulatory T cells (Tregs) play a key role in the acquisition and maintenance of peripheral tolerance [2]. In the peripheral lymphoid tissue of normal mice and humans, only 1–5% of αβ T cell receptor (TCR)+ T cells are CD4−CD8− (double-negative [DN]) T cells, capable of downregulating the immune response [3]. Adoptive transfer of allo- or xeno-antigen-activated DN T cells can prolong donor-antigen-specific skin and heart graft survival in mouse models [4–6]. Moreover, peptide-activated antigen-specific transgenic DN T cells can prevent autoimmune type 1 diabetes development [7]. Most recently, the DNCD3 splenic T cells from young NOD mice were demonstrated to be able to induce long-lasting protection against diabetes transfer into NOD/SCID mice [8].
We recently identified a new differentiation pathway by which a subset of proliferated CD4+ T cells converted to DN T cells after four to five rounds of antigen-triggered or homeostatic proliferation in vitro and in vivo. These converted DN T cells displayed a phenotype and immunosuppressive function unique from those of CD4+CD25+ Tregs, naive CD4+CD25− T cells, and activated CD4+ T cells. Moreover, using this novel pathway, tens of millions of DN T cells can be rapidly produced [9]. In this study, we sought to further test the functional potential of the converted DN T cells in an autoimmune diabetic NOD mouse model. Specifically, the ability of converted DN T cells to prevent and ameliorate autoimmune diabetes in NOD mice was investigated. Furthermore, on the basis of observations that rapamycin can selectively expand murine and human CD4+CD25+ forkhead box P3 (Foxp3)+ Tregs and concurrently inhibit effector T cell (Teff) expansion [10–12], we also report on the use of converted antigen-specific DN T cells in combination with rapamycin for the suppression of alloimmune response in vivo and the promotion of islet allograft survival in NOD recipients.
Methods
Animals
Male B6D2F1(H2b/d), C57BL/6 (H2b), and DBA/2 (H-2d) and female NOD (H-2g), NOD/SCID and NOD.Cg-Tg (NOD.BDC2.5) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in pathogen-free facilities at the University of Pittsburgh. The Institutional Animal Care and Use Committee at the University of Pittsburgh approved all animal procedures.
Conversion of NOD CD4−CD8− DN T cells in vitro
CD4+ T cells were isolated from spleens and lymph nodes of NOD mice using a T cell enrichment column (R&D Systems, Minneapolis, MN, USA) and this was followed by Ter119-, B220-, CD8-, CD11b-, TCRγδ- and NK1.1-positive cell depletion. Mature dendritic cells (mDCs) were harvested from lipopolysaccharide-stimulated bone marrow cells of NOD mice, and separated by CD86-positive selection. CD4+ T cells were cultured in 96 well round-bottom plates in complete medium (RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 10% [vol./vol.] FBS, 2 mmol/l l-glutamine and 50 μmol/l 2-mercaptoethanol) containing recombinant mouse (rm)IL-15 (100 ng/ml; Prospec-Tany, Rehovot, Israel), and stimulated with NOD mDCs at a ratio of 100,000 T cells to 25,000 DCs for up to 6 days, in the presence of GAD65 peptides or control peptides (0.1 μg/ml; GenScript Scotch Plans, NJ, USA). GAD65 peptides that encode the confined region of GAD65 included peptide 247–266 (NMYAMMIARFKMFPEVKEKG), peptide 509–528 (IPPSLRTLEDNEERMSRLSK), peptide 524–543 (SRLSKVAPVIKARMMEYGTT) and peptide 539–558 (EYGTTMVSYQPLGDKVNFFR). Control peptides that come from a non-confined region of GAD65 and do not trigger autoimmune responses included peptide 157–176 (EEILMHCQTTLKYAIKTGHP). CD4−CD8− DN fractions were isolated from mixed lymphocyte reaction (MLR) and sorted using a FACS (Aria; BD Biosciences, San Diego, CA, USA).
In vitro re-stimulation and cytokine assays
DN T cells sorted from MLR cultures were re-cultured in 96 well plates (2 × 105/well), and stimulated with anti-CD3 and anti-CD28 monoclonal antibodies (mAbs, each 2 μg/ml; BD Pharmingen, San Diego, CA, USA) for 1 or 2 days. The cytokine secretion in cell culture supernatant fractions was measured using a mouse ELISA kit (R&D Systems).
In vitro suppression assays
CD3+ T cells (1 × 105/well) from NOD.BDC2.5 mice were co-cultured in anti-CD3 mAb-coated 96 well plates with mitomycin C-treated NOD splenocytes (1 × 105/well) for 4 days. Anti-CD3 mAb was used at 1 μg/ml; mitomycin C was from Sigma-Aldrich (St Louis, MO, USA). An identical amount of DN T cells were added to MLR as regulatory cells. The cells were pulsed with [3H]thymidine (3.7 × 104 Bq/well) for the final 8 h and [3H]thymidine incorporation was measured as cpm in a liquid scintillation counter (PerkinElmer, Waltham, MA, USA).
In vivo suppression assays
Carboxyfluorescein succinimidyl ester (CFSE)-labelled C57BL/6 CD3+ T cells (20 × 106) were adoptively transferred into B6D2F1 recipients by tail-vein injection with or without 5 × 106 DN T cells (purified from MLR of DBA/2 mDCs and C57BL/6 Teffs). Recipients were treated with or without rapamycin (0.6 mg/kg; Sigma-Aldrich). Spleens were harvested 3 days after adoptive transfer, and single-cell suspensions were prepared and stained with annexin V (BD Pharmingen) for flow cytometry.
Adoptive transfer and islet transplantation
DN T cells (5 × 105) obtained from in vitro MLR with or without GAD65 peptides, as described, in combination with 5 × 105 T cells from diabetic NOD mice were transferred to NOD/SCID mice by tail-vein injection. Additionally, DN T cells (2 × 106) were transferred to 5-week-old female NOD mice. Mice were monitored for the development of diabetes by measuring blood glucose levels twice weekly. In the event of new-onset diabetes, DN T cells (4 × 106) were transferred to the new-onset diabetic NOD mice, and blood glucose levels were followed up every other day following adoptive transfer. In an islet allograft model, GAD65 peptide-primed DN T cells (5 × 106) were injected into new-onset diabetic NOD mice. On the same day, approximately 800 DBA/2 islets were transplanted under the kidney capsule of NOD mice. Recipients were treated i.p. with or without rapamycin (0.6 mg/kg, daily for 14 days). Allograft function was assessed by monitoring blood glucose levels. Diabetes or islet allograft rejection was defined as blood glucose levels of >16.7 mmol/l in two consecutive measurements.
Flow cytometric analysis
Cultured cells were harvested at various time points and analysed for the proliferation and expression of various cell surface markers. Cells were acquired on an LSRII flow cytometer (BD Biosciences) and data analysis was performed using FlowJo software (Tree star, Ashland, OR, USA).
Real-time PCR
Total RNA was extracted from cells using an RNeasy mini-kit (Qiagen, Valencia, CA, USA) and reverse transcribed to cDNA using SuperScript III RT-kit (Invitrogen, Carlsbad, CA, USA). Specific mRNA levels were quantified by real-time PCR using the ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Gene-specific primers and probes for Il2, Il4, Il17 (also known as Il17a), Foxp3, Ifnγ (also known as Ifng), Fasl, granzyme B (GZMB) and perforin (PRF) were purchased from Applied Biosystems. Data are expressed using the Ct method and normalised to the housekeeping gene Gpdh.
Statistical analysis
Analyses for statistically significant differences were performed using the Student’s t test and one-way ANOVA test. The influence of different treatments on islet allograft survival was analysed using a logrank test; p < 0.05 was considered significant.
Results
DN T cells can be converted from NOD CD4+ T cells in syngeneic MLR
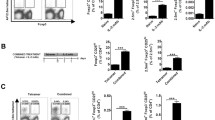
To explore the potential application of DN T cells converted from the CD4+ T cells of a NOD host to block autoimmune diabetes in NOD mice, CFSE-labelled CD4+ T cells isolated from spleen and lymph nodes of female NOD mice were co-cultured with syngeneic bone marrow mDCs plus rmIL-15. After 6 days in vitro MLR, approximately 50% of NOD CD4+ T cells lost CD4 expression (Fig. 1a, b). Consistent with our previous report, the converted DN T cells expressed a unique set of cell surface markers: αβTCR+CD4−CD8−B220+CD44+CD69+NK1.1− (Fig. 1c–j). Additionally, compared with NOD CD4+ T cells, converted DN T cells expressed undetectable Foxp3, Il2 and Il4 mRNA levels, very low Il17 mRNA levels, but high Ifnγ, Fasl, GZMB and PRF levels (Fig. 1k–m). Upon re-stimulation with anti-CD3/CD28 mAb, IL-2 and IL-4 levels in the converted DN T cell culture supernatant fractions were significantly lower, while IFN-γ production was higher than that in the CD4+ T cells (Fig. 1n–p). In addition, the converted DN T cells maintained a stable CD4− phenotype and low proliferation after re-stimulation, as previously reported by Zhang et al. [9], consistent with anergic properties ascribed to DN T cells.
NOD CD4+ T cells could be converted to DN T cells via NOD mDCs and IL-15 stimulation in vitro. a CFSE-labelled CD4+ T cells from NOD mice had prominent proliferation after 6 days of culture with NOD mDCs and rmIL-15 (100 ng/ml). b Flow cytometry analysis showed a high percentage of CD4+ T cells lost their CD4 expression and became DN T cells. The square shows 51.9% of cells in the designated gate. DN T cells (grey histograms) have a unique set of cell-surface markers, which are CD3 (c), TCRβ (d), CD44 (e), CD69 (f) and B220 (g), but do not express CD4 (h), CD8 (i) or NK1.1 (j) . Mouse isotype Ig used as control (white histograms). Converted DN T cells (grey bars) expressed undetectable Foxp3 (l), Il2 (k) and Il4 (k) mRNA, very low Il17 (l), but high Ifnγ (k), Fasl (m), GZMB (m) and PRF (m) mRNA levels compared with NOD CD4+ T cells (black bars). IL-2 (n) and IL-4 levels (o) in the culture supernatant fractions of converted DN T cells (grey bars) were lower, while IFN-γ level (p) was higher than that in the CD4+ T cells (black bars) upon re-stimulation with anti-CD3/CD28 mAb. Results shown here represent three independent experiments
The addition of GAD65 peptides in the initial MLR enhances regulatory function of DN T cells
As GAD65 is a putative self-antigen produced by beta cells and plays a critical role in the initial events in type 1 diabetes [13, 14], synthetic peptides derived from a GAD65 isoform were added in the initial MLR to induce beta cell-specific DN T cells. Isolated CD4+CD25− T cells from female NOD mice were cultured with syngeneic mDCs plus rmIL-15, with or without GAD65 peptides. After 6 day co-culture, DN T cells were isolated from the MLR. The effects of DN T cells on beta cell antigen-specific immune responses were then examined by NOD.BDC2.5 CD3+ T cell proliferation assay. As shown in Fig. 2a, DN T cells converted from NOD CD4+ T cells, with or without GAD65 peptides, significantly inhibited the NOD.BDC2.5 CD3+ T cell proliferation triggered by anti-CD3 mAb and mitomycin-C-treated NOD splenocytes (p < 0.001); however, GAD65-peptide-primed DN T cells demonstrated greater suppressive function than that achieved without GAD65 (DN vs GAD65-DN, p < 0.001). DN T cells themselves were hyporesponsive to the stimulation by anti-CD3 mAb and splenocytes.
NOD CD4+ T cell-converted DN T cells could suppress syngeneic T cell proliferation, and GAD65 peptide in the initial MLR enhanced the inhibition of DN T cells both in vitro and in vivo. a CD3+ T cells from NOD BDC2.5 mice were cultured for 4 days in the presence of anti-CD3 mAb and mitomycin C-treated NOD splenocytes, and treated with the converted DN T cells, with or without GAD65 priming. T cell proliferation was measured by [3H]thymidine incorporation. Results are presented as mean cpm ± SD. Data are representative of three experiments with similar results. b DN T cells obtained from in vitro MLR, with or without GAD65 priming, in combination with T cells from diabetic NOD mice at a 1:1 ratio were transferred to NOD/SCID mice by tail-vein injection. Mice were monitored for the development of diabetes by examining blood glucose levels. Adoptive transfer of diabetogenic T cells alone (crosses, n = 5) led to a rapid onset of diabetes in all NOD/SCID recipients. The co-transfer of DN T cells with diabetogenic T cells (upright triangles, n = 4) delayed the onset of diabetes and the DN T cells primed with GAD65 (reverse triangles, n = 4) further enhanced the protection from diabetes onset. All recipients who received DN T cells (squares, n = 4), with or without GAD65 priming (circles, n = 4), remained diabetes free for more than 25 weeks post adoptive transfer
NOD DN T cells block autoimmune diabetes and GAD65 peptide priming enhances the efficacy of blockade
The functional potential of the converted DN T cells in autoimmune diabetes was further tested in an adoptive transfer model in NOD/SCID recipients. The adoptive transfer of T cells from autoimmune diabetic NOD mice precipitated a rapid onset of diabetes within 12 weeks following adoptive transfer in all NOD/SCID recipients (Fig. 2b). In contrast, all NOD/SCID recipients that received DN T cells alone, which were converted from CD4+ T cells of diabetic NOD mice either with or without GAD65 peptide in MLR, remained diabetes free for more than 25 weeks post adoptive transfer. The co-transfer of DN T cells with diabetogenic T cells at a 1:1 ratio delayed the onset of diabetes in NOD/SCID mice. Moreover, co-transfer of GAD65-primed DN T cells with diabetogenic T cells demonstrated further enhanced efficacy in significantly delaying the onset of diabetes in NOD/SCID mice (T vs T+DN [GAD65], p < 0.01). Thus, the DN T cells converted from diabetogenic CD4+ T cells of diabetic NOD mice were capable of suppressing autoimmunity, and GAD65 peptide priming further enhances DN T cell efficacy.
Histopathological changes were assessed in haematoxylin and eosin (H&E)-stained pancreas sections from adoptively transferred NOD/SCID recipients at week 5 after cell transfer. A massive mononuclear cell infiltration of the islets with loss of islet structure was observed in NOD/SCID recipients that received T cells from diabetic NOD donors (Fig. 3a). In contrast, pancreas harvested from NOD/SCID recipients given DN T cells alone (either with or without GAD65 priming) (Fig. 3c, e) or in combination with diabetogenic T cells (Fig. 3b, d) showed normal islet structure without obvious mononuclear cell infiltration, suggesting that DN T cells converted from diabetogenic CD4+ T cells possess the ability to suppress diabetogenic T cells and prevent autoimmune disease in vivo.
Histological analysis of islets from different adoptively transferred NOD/SCID recipients. Routine H&E staining of pancreas isolated 5 weeks after adoptive transfer. Massive tissue infiltration by mononuclear cells with destruction of islets is observed in mice receiving diabetogenic T cells alone (a). Recipients receiving diabetogenic T cells plus DN T cells (b), DN T cells alone (c), diabetogenic T cells and GAD65-DN T cells (d) or GAD65-DN T cells alone (e) show intact islets with minimal mononuclear cell infiltration. Paraffin sections, original magnification × 100
Adoptive transfer of DN T cells can prevent diabetes development and reverse new-onset diabetes
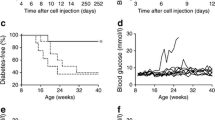
To explore the potential application of DN T cells in preventing type 1 diabetes, DN T cells (converted from CD4+CD25− T cells of NOD mice by co-culture with NOD mDCs, rmIL-15 and GAD65 peptide in MLR for 6 days) were adoptively transferred into 5-week-old prediabetic NOD female mice. The administration of single dose of 2 × 106 DN T cells resulted in a significant delay of diabetes onset from 11 weeks post adoptive transfer in the untreated group to 17 weeks post adoptive transfer in the DN treated group. At 25 weeks post adoptive transfer, 85% of the untreated NOD mice developed diabetes, whereas only 25% of the NOD mice given GAD65-primed DN T cells therapy became diabetic (Fig. 4a). Reversing new-onset autoimmune diabetes in NOD mice is a more daunting challenge. Our results show that a single transfer of 4 × 106 DN T cells slightly, but significantly, postponed the progression of hyperglycaemia in new-onset diabetic NOD recipients. Blood glucose levels of mice treated with DN T cells were significantly lower than those of the control groups, but there was no reversal of hyperglycaemia. However, a single transfer of an equivalent amount of GAD65-primed DN T cells into new-onset diabetic NOD mice resulted in reversal of hyperglycaemia in all recipients within 12 days. Two recipients remained diabetes free over 3 months. The rest of the recipients showed transient reversal of autoimmune diabetes; blood glucose levels started to rise after 12 days of adoptive transfer (Fig. 4b).
Adoptive transfer of DN T cells could prevent diabetes development and reverse new onset of diabetes. a A single transfer of 2 × 106 GAD65-primed DN T cells (triangles) into 5-week-old female NOD recipients (circles, NOD control) significantly delayed the onset of diabetes in NOD mice (p = 0.0162). b A single transfer of 4 × 106 DN T cells showed the trend of lowering blood glucose levels in new-onset diabetic NOD mice. Blood glucose levels of GAD65-DN T cell-treated mice (reverse triangles, n = 6) were significantly lower than those of the control group (squares, n = 4) and the DN T cell-treated group (upright triangles, n = 6) on day 12 (p < 0.001)
DN T cells act in conjunction with rapamycin to inhibit alloimmune response, promote T cell apoptosis and prolong islet allograft survival
To examine the influence of DN T cells combined with rapamycin on the proliferation of alloreactive T cells in vivo, CFSE-labelled C57BL/6 CD3+ T cells were adoptively transferred into B6D2F1 recipients. Flow cytometry analysis revealed that both CD4+ and CD8+ T cells from C57BL/6 mice exhibited robust proliferation after 3 days of adoptive transfer in B6D2F1 recipients. DN T cell treatment alone did not significantly inhibit T cell proliferation. Administration of rapamycin at a dose of 0.6 mg/kg partially inhibited both CD4+ and CD8+ T cell proliferation, though greater inhibition of CD8+ T cell proliferation was observed. However, the combination of DN T cells and rapamycin exerted more potent suppression of both CD4+ and CD8+ T cell proliferation than that achieved with rapamycin alone (Fig. 5a–h). Moreover, combined treatment of DN T cells with rapamycin significantly increased annexin V staining for both CD4+ and CD8+ T cells (Fig. 5i–p), suggesting an enhanced apoptosis of alloreactive T cells in vivo.
The influence of DN T cells combined with rapamycin on the alloreactive T cell proliferation and apoptosis. CFSE-labelled T cells were analysed for proliferation index (PI) or annexin V production by flow cytometry at day 3 of adoptive transfer. Both CD4+ (a) and CD8+ (e) T cells from C57BL/6 mice had robust proliferation after 3 days of adoptive transfer in B6D2F1 recipients. DN T cells alone did not significantly affect CD4+ (c) or CD8+ (g) T cell proliferation. Rapamycin (Rapa) partially suppressed the proliferation of both CD4+ (b) and CD8+ (f) T cells. Co-transfer of DN T cells further enhanced the suppression of both CD4+ (d) and CD8+ (h) T cells in vivo by rapamycin. Combined treatment of rapamycin and DN T cells significantly induced annexin V staining for both CD4+ (i–l) and CD8+ (m–p) T cells in vivo
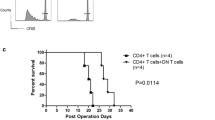
In order to evaluate the influence of DN T cells and rapamycin on allograft survival, MHC mismatched DBA/2 islet allografts were transplanted into diabetic NOD mice. As shown in Fig. 6, untreated recipients rejected their grafts in a mean survival time (MST) of 6 days (n = 4). Administration of rapamycin or DN T cells alone did not significantly delay islet allograft rejection (MST, 8 day; n = 5). In contrast, single transfer of 5 × 106 GAD65-primed converted DN T cells plus 14 day rapamycin treatment significantly prolonged islet allograft survival (MST, 40 days, p = 0.002) and proved more effective in promoting engraftment than the two monotherapies (p < 0.01).
Survival of islet allografts in response to a combined treatment of DN T cells and rapamycin. Diabetic NOD mice were transplanted with a DBA/2 islet allograft under the renal capsule and treated with DN T cells (diamonds, n = 5) or rapamycin alone (upright triangles, n = 5) or in combination (reverse triangles, n = 5), as indicated in the Methods (squares, no treatment control, n = 4). Combined treatment with DN T cells and rapamycin resulted in a significantly prolonged DBA/2 islet survival time in diabetic NOD recipients (p = 0.0079)
Discussion
Tregs have emerged as being critically important for the control of autoimmunity and for the maintenance of allograft tolerance [15, 16]. As the elucidation of the immunoregulatory properties of CD4−CD8− DN T cells in T cell-mediated immune response, it has been anticipated that adoptive cell therapy using DN T cells could become an attractive treatment for autoimmune disease and transplant rejection [3, 4]. Nevertheless, the lack of precise markers, sufficient number and antigen specificity of DN T cells are major hurdles for their in vivo application.
We have identified a new differentiation pathway in a normal mouse model for the conversion of mouse peripheral CD4+ T cells to DN regulatory T cells. These converted DN T cells were resistant to activation-induced cell death (AICD) and were highly potent in suppressing alloimmune responses both in vitro and in vivo in an antigen-specific manner [9]. In this study, we further demonstrated the conversion of CD4+ T cells isolated from NOD mice with the stimulation of syngeneic mDCs plus rmIL-15 in vitro. After 6 days MLR, approximately 50% of the proliferated T cells were identified as DN T cells by expressing αβTCR+CD4−CD8−B220+CD44+CD69+NK1.1−. Converted DN T cells express high levels of Fasl gene and cytotoxic lymphocyte-related gene GZMB and PRF, but undetectable amounts of Foxp3, Il2, Il4 and Il17. In addition, the re-stimulation of DN T cells did not significantly change the phenotype and the cytokine profile of DN T cells. As shown in Fig. 1n–p, the IL-2 and IL-4 production was lower, while IFN-γ was higher in DN T cell culture supernatant fractions after re-stimulation in comparison with that of CD4+ T cells. This finding provides the feasibility of NOD CD4+-converted DN T cells to be used in autoimmune diabetic NOD model and suggests that this cell population is uncommon to other Tregs subsets and cytotoxic features may contribute at least partially to their unique regulatory ability.
Studies have revealed that the beta cell antigen-specific Tregs are more potent in blocking autoimmune diabetes, even in a highly diabetes-prone Treg-deficient CD28KO NOD model [17]. GAD has come under the scrutiny of diabetes researchers because some of the earliest auto-antibodies found in prediabetic patients are GAD specific [18, 19]. There is also evidence that the presence of GAD protein in beta cells is strictly correlated with the development of diabetes in NOD mice and that GAD is the essential auto-antigen to initiate the disease by activating GAD-specific T cells. Additionally, when transplanted into diabetic NOD mice, islet cells unable to produce GAD, but not normal islet cells, were spared from immune attack [20]. As the confined region of GAD65 that triggers initial autoimmune responses has been identified [13], we employed synthetic GAD65 peptides for the induction of beta cell antigen-specific DN T cells and hypothesised that DN T cells induced by syngeneic mDCs plus GAD65 peptide would possess beta cell antigen specificity and the ability to suppress autoimmunity. Indeed, the addition of GAD65 peptides in the MLR had a similar effect on the conversion rate, cell surface phenotype and gene expression profile of converted DN T cells (data not shown) as that without GAD65 peptide treatment. To test the regulatory function of converted DN T cells on beta cell antigen-specific T cell immune responses, we used NOD.BDC2.5 mice, which carry both rearranged Tcrα (also known as Tcra) and Tcrβ (also known as Tcrb) genes from the diabetogenic H2-Ag7 restricted BDC2.5 CD4+ T cell clone [21]. Our results show that the NOD CD4+ converted DN T cells significantly suppressed anti-CD3 and syngeneic antigen presenting cells (APCs) triggered NOD.BDC2.5 T cell proliferation, in which the DN T cells primed with GAD65 peptides exerted much more potent suppressive ability, demonstrating the effect of antigen-specific DN T cells on autoimmune response.
The functional potential of the converted DN T cells on autoimmune diabetes was further demonstrated in an adoptive transfer model in NOD/SCID recipients. The co-transfer of an equal number of DN T cells with diabetogenic NOD T cells resulted in a delay of diabetes onset in NOD/SCID recipients, and the GAD65-primed DN T cells further delayed autoimmune diabetes compared with the NOD T cells transfer group, accompanied by a marked decrease in pancreas mononuclear cell infiltration at 5 weeks after adoptive cell transfer. Furthermore, the adoptive transfer of a single dose of 2 × 106 DN T cells into 5-week-old NOD hosts resulted in the significant delay and reduced the incidence of diabetes onset. This finding supports the idea that the application of ex vivo CD4+ converted DN T cells is beneficial to prevent autoimmune diabetes in the NOD model and that peptide-activated antigen-specific DN T cells can exert a more potent effect in vivo. Thus, the inhibition of the homing, proliferation and effect function of islet-destructive T cells may at least in part underlie the delayed onset of autoimmune diabetes induced by converted DN T cells. We did not study the homing of DN T cells in this study because of the lack of a proper tracking marker. We have previously analysed the homing of the converted C57BL/6 DN T cells after their adoptive transfer to syngeneic Rag-1 knockout mice, which were then challenged with the DBA/2 skin allograft. We found that CD3+ T cells homed into recipients’ spleen, mesenteric lymph nodes and draining lymph nodes and 60–80% CD3+ T cells remained CD4−CD8− at 100 days post transplantation (data not shown). However, the homing and function of DN T cells in vivo warrant further investigation.
Although numerous treatments that prevent the development of diabetes and progression of the autoimmune ‘invasive insulitis’ in prediabetic NOD mice have been identified, far more challenging is the restoration of a euglycaemic state in mice with frank type 1 diabetes [22]. The infusion of islet-specific Tregs, which were isolated from NOD.BDC2.5 mice and were expanded with antigen-pulsed DCs and IL-2 in vitro, was effective in inducing long-lasting reversal of hyperglycaemia in 50% of mice in which overt diabetes had developed [23]. In this study, we assessed the ability of converted DN T cells to treat new-onset diabetic NOD recipients. A single transfer of 4 × 106 DN T cells significantly postponed the progression of hyperglycaemia, though there was no reversal of hyperglycaemia. However, a single transfer of the same number of GAD65-primed DN T cells resulted in reversal of hyperglycaemia in all recipients within 12 days. Two recipients remained diabetes free over 3 months. The rest of the recipients showed transient reversal of autoimmune diabetes: blood glucose levels started to rise after 12 days of adoptive transfer. These results suggest the favourable influence of antigen-specific DN T cells on the treatment of new-onset autoimmune diabetes. Therefore, our data show the promise of DN T cell-based therapy for improvement in autoimmune diabetes.
Islet transplantation offers hope for patients with type 1 diabetes, with physiologically regulated insulin release by islet allograft preventing diabetes-related complications. However, transplantation of islet allografts into spontaneously diabetic NOD hosts provides an even more daunting challenge because of the generalised defect in tolerance mechanisms and the presence of pre-activated cytopathic islet-specific autoimmune T cells [24, 25]. Rapamycin is a macrolide antibiotic with potent immunosuppressive and immunoregulatory properties. The mammalian target of rapamycin has an important role in modulating innate and adaptive immunity [26]. In this study, we have demonstrated that converted DN T cells and rapamycin act in concert in vivo to inhibit the proliferation and promote the apoptosis of both CD4+ and CD8+ T cells. This suggests potential interaction between DN T cells and rapamycin-sensitive mammalian target of rapamycin signalling pathways in T cell-cycle progression. However, DN T cells by themselves do not have a significant effect on CD4+ or CD8+ T cell proliferation and apoptosis induction, which differed from the effects previously observed in vitro or in vivo [9, 27]. This discrepancy might be due to the complexity of alloimmune responses in vivo, which depend on the extent of immune stimulation and the sufficiency of DN T cell-mediated regulation. In an adoptive transfer model, the naive T cells from C57BL/6 mice encountered alloantigens of MHC-mismatched B6D2F1 recipients both by direct and indirect antigen-presentation pathways and resulted in a robust T cell activation and proliferation as shown in Fig. 5. It is not a surprise that adoptive transfer of DN T cells alone did not have a significant effect on alloreactive CD4+ and CD8+ T cell proliferation demonstrated by both CFSE-assisted assay and islet allograft survival, because the more rapid expansion of alloaggressive T cells overcomes the protective effects of DN regulatory T cells. In contrast, the effective immunosuppressive rapamycin therapy that significantly reduced the alloreactive CD4+ and CD8+ T cells allowed DN regulatory T cells to exert their suppressive effects. This is consistent with previous reports [28–30]. We further assessed whether combined DN T cells and rapamycin treatment can promote islet allograft engraftment in MHC-mismatched pancreatic islet allotransplantation in autoimmune NOD recipients. Indeed, the single-dose transfer of DN T cells plus 14 days of rapamycin administration achieved significant prolongation of islet allograft survival compared with any of the monotherapies, indicating the beneficial role of the DN T cell-based immunoregulatory strategy in NOD islet allograft engraftment. The concerted influence of these two components on autoimmune T cell activity and AICD may ensure effective control of anti-donor reactivity and promote allograft survival. However, further tailoring the dose and/or frequency of GAD65-primed DN T cells in concomitant regimens will be required for optimal therapeutic effect in type 1 diabetes and islet allotransplantation.
In short, this study demonstrates that beta cell antigen-specific DN T cells can be induced from prediabetic NOD CD4+ T cells in vitro, efficiently prevent the onset and progress of autoimmune diabetes in vivo, and also act in conjunction with rapamycin to promote islet allograft survival in NOD mouse models. The isolation of tens of millions of highly purified CD4+ T cells from the peripheral blood of a patient is within reach of current clinical technology. By stimulating highly purified CD4+ T cells with syngeneic or allogeneic bone marrow mDCs or APCs plus rmIL-2 and/or rIL-15, it is possible to convert sufficient antigen-specific DN T cells within a few days. Our results using DN T cells converted from prediabetic NOD CD4+ T cells in adoptive transfer and islet transplantation models shows a promising cell-based strategy for treatment of autoimmune diabetes and its feasibility in clinical application.
Abbreviations
- AICD:
-
Activation-induced cell death
- APC:
-
Antigen presenting cell
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- DN T cell:
-
CD4−CD8− double-negative T cell
- FoxP3:
-
Forkhead box P3
- H&E:
-
Haematoxylin and eosin
- mAb:
-
Monoclonal antibody
- mDC:
-
Mature dendritic cell
- MLR:
-
Mixed lymphocyte reaction
- MST:
-
Mean survival time
- rm:
-
Recombinant mouse
- TCR:
-
T cell receptor
- Teff:
-
Effector T cell
- Treg:
-
Regulatory T cell
References
Rossini AA, Mordes JP, Like AA (1985) Immunology of insulin-dependent diabetes mellitus. Annu Rev Immunol 3:289–320
Sakaguchi S, Sakaguchi N, Shimizu J et al (2001) Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182:18–32
Thomson CW, Lee BP, Zhang L (2006) Double-negative regulatory T cells: non-conventional regulators. Immunol Res 35:163–178
Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L (2000) Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med 6:782–789
Chen W, Zhou D, Torrealba JR, Waddell TK, Grant D, Zhang L (2005) Donor lymphocyte infusion induces long-term donor-specific cardiac xenograft survival through activation of recipient double-negative regulatory T cells. J Immunol 175:3409–3416
Ma Y, He KM, Garcia B, Min W, Jevnikar A, Zhang ZX (2008) Adoptive transfer of double negative T regulatory cells induces B cell death in vivo and alters rejection pattern of rat-to-mouse heart transplantation. Xenotransplantation 15:56–63
Ford MS, Chen W, Wong S et al (2007) Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. Eur J Immunol 37:2234–2241
Duncan B, Nazarov-Stoica C, Surls J et al (2010) Double negative (CD3+ 4– 8−) TCR alphabeta splenic cells from young NOD mice provide long-lasting protection against type 1 diabetes. PLoS One 5:e11427
Zhang D, Yang W, Degauque N, Tian Y, Mikita A, Zheng XX (2007) New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood 109:4071–4079
Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG (2006) Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177:8338–8347
Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I (2006) Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107:1018–1023
Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A (2007) Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol 178:320–329
Kaufman DL, Clare-Salzler M, Tian J et al (1993) Spontaneous loss of T cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366:69–72
Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO (1993) Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366:72–75
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Adeegbe D, Bayer AL, Levy RB, Malek TR (2006) Cutting edge: allogeneic CD4+CD25+Foxp3+ T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J Immunol 176:7149–7153
Tang Q, Henriksen KJ, Bi M et al (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199:1455–1465
Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A (1982) Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 298:167–169
Harrison LC, Honeyman MC, DeAizpurua HJ et al (1993) Inverse relation between humoral and cellular immunity to glutamic acid decarboxylase in subjects at risk of insulin-dependent diabetes. Lancet 341:1365–1369
Yoon JW, Yoon CS, Lim HW et al (1999) Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science 284:1183–1187
Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB (2001) Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol 166:908–917
Shoda LK, Young DL, Ramanujan S et al (2005) A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 23:115–126
Tarbell KV, Petit L, Zuo X et al (2007) Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med 204:191–201
Maki T, Ichikawa T, Blanco R, Porter J (1992) Long-term abrogation of autoimmune diabetes in nonobese diabetic mice by immunotherapy with anti-lymphocyte serum. Proc Natl Acad Sci USA 89:3434–3438
Noorchashm H, Moore DJ, Noto LE et al (2000) Impaired CD4 T cell activation due to reliance upon B cell-mediated costimulation in nonobese diabetic (NOD) mice. J Immunol 165:4685–4696
Thomson AW, Turnquist HR, Raimondi G (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9:324–337
Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L (2002) The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. J Exp Med 196:261–267
Zheng XX, Sanchez-Fueyo A, Domenig C, Strom TB (2003) The balance of deletion and regulation in allograft tolerance. Immunol Rev 196:75–84
Li XC, Strom TB, Turka LA, Wells AD (2001) T cell death and transplantation tolerance. Immunity 14:407–416
Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX (2002) Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol 168:2274–2281
Acknowledgements
The study was partially supported by grants 1-2005-1001 (to X. X. Zheng) and 3-2008-134 (to W. Zhang) from the Juvenile Diabetes Foundation International and a grant (to D. Zhang) from the Plastic Surgery Educational Foundation.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Zhang and W. Zhang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, D., Zhang, W., Ng, T.W. et al. Adoptive cell therapy using antigen-specific CD4−CD8− T regulatory cells to prevent autoimmune diabetes and promote islet allograft survival in NOD mice. Diabetologia 54, 2082–2092 (2011). https://doi.org/10.1007/s00125-011-2179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2179-4