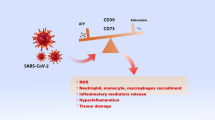
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has significantly impacted the world and has driven many researchers into the pathophysiology of COVID-19. In the findings, there is a close association between purinergic signaling and the immune response. Then, this study aimed to evaluate alterations in the purinergic signaling in COVID-19 patients according to range severity. We divided the COVID-19 patients into moderate and severe cases following the guideless of NIH and WHO, together with clinical characteristics. The blood samples were collected to obtain PBMCs and platelets. We analyzed the ectonucleotidase activities through ATP, ADP, AMP, Ado hydrolysis, E-NTPDase1 (CD39), and 5′-NT (CD73) expression by flow cytometry in total leukocytes. The extracellular ATP was measured by bioluminescence, and cytokines were analyzed by flow cytometry. We observed a decrease in ATP hydrolysis and increased AMP hydrolysis in PBMCs for both groups. In severe cases, ATP hydrolysis was raised for the platelets, while ADP and AMP hydrolysis have risen significantly in both groups. Additionally, there was a significant increase in ADP hydrolysis in severe cases compared to moderate cases. In addition, we observed an increase in the ADA activity in platelets of moderate patients. Moderate and severe cases showed increased expression of CD39 and CD73 in total leukocytes. To finalize the purinergic signaling, extracellular ATP was increased in both groups. Furthermore, there was an increase in IL-2, IL-6, IL-10, and IL-17 in moderate and severe groups. Thus, for the first time, our findings confirm the changes in purinergic signaling and immune response in COVID-19, in addition to making it more evident that the severity range directly impacts these changes. Therefore, the therapeutic potential of the purinergic system must be highlighted and studied as a possible target for the treatment of SARS-CoV-2 disease.
Key messages
-
COVID-19 patients exhibit alterations in purinergic system and immune response.
-
High levels of extracellular ATP lead to different inflammatory responses.
-
CD39 and CD73 expression were increased in COVID-19 patients.
-
Cytokines IL-2, IL-6, IL-10, and IL-17 also were altered in these patients.
-
The purinergic system may be a possibility target to SARS-CoV-2 treatments.
Graphical abstract

Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) is an ongoing global pandemic of infectious disease identified initially in December 2019 in Wuhan, China. The outbreak of respiratory cases culminated in the officially declared pandemic on March 11, 2020, and caused more than 4 million confirmed deaths worldwide until July 14, 2021. The etiological agent of the disease is the newly identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a beta coronavirus of the Coronaviridae family, transmitted through contact hand-mouth-nose-eye with contaminated droplets expelled by coughing and sneezy [1, 2]. Mild symptoms may include fatigue, headache, fever, cough, myalgia, diarrhea, anorexia, and dyspnea. However, severe cases may evolve to pulmonary edema due to pneumonia, presenting hypoxemia and coagulopathy, eventually leading to organ failure and death [3,4,5,6].
As the first line of defense against pathogens, the host innate immune system promptly recruits immune cells to fight viral infections. The course of inflammatory reactions may influence disease outcomes [7]. Evidence suggests that COVID-19 patients may present an immune dysregulation with high infiltration of inflammatory cells, such as neutrophils and monocytes/macrophages, increasing blood levels of IL-1β, IL-6, IL-2 IL-7, and TNF-α, among other proinflammatory cytokines and chemokines [8,9,10]. Although host inflammatory processes triggered by SARS-CoV-2 infection are essential for the virus-fighting, excessive and prolonged responses can result in a cytokine storm (CS), leading to multiorgan damage [11, 12].
The ubiquitous presence of purinergic components in almost all body tissues and its close relationship with the immune system highlights the purinergic system as a feasible candidate cellular signaling pathway associated with disease severity and progression in COVID-19 patients. This sophisticated cell–cell communication system orchestrates numerous cellular responses in the context of health and disease, displaying immunomodulatory capabilities and widely influencing cellular proliferation, differentiation, and death processes. Extracellular signaling molecules include nucleotides such as adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and the nucleoside adenosine (Ado), implicated in several pathophysiological events [13,14,15,16,17].
The levels of purinergic molecules are controlled by a complex network of nucleotide/nucleoside enzymes, collectively known as ectonucleotidases, expressed on the surface of cells. The presence of these molecules in the extracellular milieu can rapidly activate the nucleoside triphosphate diphosphohydrolase 1 (NTPDase-1/CD39), 5′-nucleotidase (5'-NT/CD73), and adenosine deaminase (ADA) enzymes, which metabolize ATP/ADP into AMP, AMP into adenosine (Ado), and finally this into inosine, respectively [18,19,20,21].
A robust body of evidence supports the involvement of purinergic signaling in several disease conditions, such as cancer [17, 22,23,24,25,26,27,28], inflammatory process [29], diabetes, and hypertension [30, 31]. Recently, studies have hypothesized the potential role of purinergic signaling in COVID-19 [32,33,34,35]. This assumption is supported mainly by the role of the purinergic signaling molecules such as ATP as a damage-associated molecular pattern (DAMP) in the extracellular environment [36]. During SARS-CoV-2 infection, the active replication and release of the virus can cause the host cells to undergo pyroptosis, promoting the outpour of ATP, which acts as a signaling molecule activating the purinergic system components such as enzymes and receptors. This process triggers downstream signaling cascades that exacerbate the immune responses and further increase the inflammatory process [11, 37].
Therefore, considering the possible role of the purinergic system in the pathogenesis caused by SARS-CoV-2 and its correlation with the immune system, we aimed to evaluate alterations in the purinergic signaling in COVID-19 patients to range severity.
Materials and methods
Ethical statement
The Institutional Ethics Committee approved this project of the Federal University of the South Frontier—Chapecó Campus (Chapecó, Santa Catarina State, Brazil), under registration number 4.333.241. All the protocols performed in this investigation followed the ethical standards of the institutional and national research committee on the involvement of human participants. Patients and control group (CT) were admitted to the study after signing the informed consent form.
Materials, reagents, and equipment
This study was carried out with reagents and chemicals of analytical grade purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA) and Merck KGaA (Darmstadt, Germany). All other reagents otherwise not stated were of chemical purity. All measurement analyses were performed using a Multiskan™ GO microplate spectrophotometer (Thermo Fisher Scientific, USA). Flow cytometry analysis was carried out in an Accuri™ C6 Plus cytometer (BD Biosciences) and analyzed by the FlowJo V10 software.
Study design, patients’ selection criteria, and disease severity classification
A cross-sectional study of 62 hospitalized patients diagnosed with COVID-19 from the city of Chapecó (Santa Catarina State, Brazil) was conducted between March and April 2021. Male and female patients (age ≥ 18 years) were enrolled in this study. The diagnosis of SARS-CoV-2 infection was assessed by collecting biological material by nasopharyngeal swab and confirmed by RT-PCR. Besides, a total of 49 healthy subjects—CT, male and female (age ≥ 18 years), and without any symptoms of flu syndrome—were selected for this investigation. The negativity of SARS-CoV-2 infection in the CT was assessed by RT-PCR analysis after nasopharyngeal swab collection. CT subjects did not present any history of non-communicable diseases (NCDs), such as diabetes, hypertension, cancers, cardiovascular diseases (such as stroke and heart attacks), and respiratory diseases (such as asthma and chronic obstructive pulmonary disease). Figure 1 represents the experimental design, and in Table 1 are expressed clinical and demographic characteristics of patients and CT.
Schematic representation of the study design. A total of 111 volunteers, including 49 healthy subjects (control group) and 62 SARS-CoV-2 patients (COVID-19 group), were enrolled in the study. Upon the collection of biological samples for the biochemical analysis, infection was assessed using RT-PCR diagnosis. Blood samples were obtained by venipuncture from control subjects and COVID-19 patients and processed to obtain serum, PMBCs, and platelets according to the requirements of each assay. Purinergic enzyme activities were performed by using PMBCs and platelets as samples. Cytokines levels were assessed by using serum samples
Looking to understand whether disease severity categories interfere both in purinergic signaling, we divided the confirmed COVID-19 patients into moderate and severe cases following the COVID-19 Treatment Guidelines Panel by the National Institutes of Health [38] and the Guidelines for Novel Coronavirus Clinical Manifestations by the World Health Organization [39], together with clinical characteristics available in Table 1, such as outcome, type of admission, and use of mechanical ventilation (MV). Thus, considering the guidelines definitions, patients with a better outcome, without intensive care unit (ICU) admission, and MV were considered moderate cases, representing 40 patients. In contrast, severe cases were the worst outcome, ICU admission, and MV necessity, representing 22 patients.
Blood collection and isolation of blood components
After all of the patients or familiar enrolled in this study provided written informed consent, whole blood was obtained by venipuncture to isolate the components required for later analysis according to particular requirements for each protocol. We collected a total of 15 mL of blood using a BD Vacutainer® (BD Biosciences, San Diego, CA, USA) tube with an EDTA system for isolation of peripheral blood mononuclear cells (PBMCs) and separated on Ficoll-Histopaque density gradients, following the general guidelines described [40] with adaptations. Briefly, the EDTA tube containing whole blood was centrifuged at 3500 rpm for 15 min for separation in plasma, buffy coat, and red blood components. In sequence, the buffy coat (rich in PBMCs) was diluted 1:1 with phosphate-buffered saline (PBS), carefully layered onto Ficoll-Histopaque, and centrifuged at 1800 rpm for 30 min. Isolated PBMCs were carefully collected (2–4 mL), resuspended in 15 mL PBS, and centrifuged at 1500 rpm for 15 min. When necessary, the PBMCs were washed with a hemolytic buffer solution to eliminate red blood cell (RBC) residues and centrifuged at 1500 rpm for 15 min. Then, the supernatant was removed, the pellet was resuspended in 15 mL PBS and centrifuged at 1500 rpm for 10 min. Finally, the supernatant was removed, and the pellet was resuspended in 1 mL of saline solution (NaCl, 0.9%) to assess nucleotide/nucleoside hydrolysis and 1 mL RPMI medium (11.1 mM glucose, supplemented with 3% FBS, 50 units/ml penicillin, 50 g/mL streptomycin) with 10% of dimethyl sulfoxide (DMSO) to further analysis. The platelet separation occurred according to the previous method with modifications [41, 42]. For this, a total blood was collected with sodium citrate as anticoagulant and centrifuged at 1500 rpm for 10 min. After, the platelet-rich plasma was centrifuged at 5000 rpm for 30 min and washed with 3.5 mM HEPES buffer, pH 7.0 at least twice. The platelet pellets were suspended in HEPES buffer.
Protein determination
The proteins were determined according to the method of [43] and adjusted in the ranges of 0.1–0.2 for samples of PBMCs and 0.4–0.6 mg/mL for platelets, using saline solution when necessary.
Extracellular quantification of ATP
The quantitative ATP determination was developed using commercial kit (Invitrogen®) by bioluminescence assay with recombinant firefly luciferase and its substrate D-luciferin in serum of COVID-19 patients and CT group. The assay is based on luciferase’s requirement for ATP in producing light (emission maximum ~560 nm at pH 7.8 [44]. We combined the reaction components as follows to make a standard reaction solution and adjust the volumes according to particular requirements. After a 15-min incubation, luminescence was measured and represented as nM/ATP.
E-NTPDase1 (CD39), E-5′-NT (CD73), and ADA purinergic enzymes activities
The activities of purinergic enzymes were performed by using platelets and PBMCs samples of CT and COVID-9 patients. The hydrolysis of nucleotides such as ATP/ADP (E-NTPDase1, CD39), AMP (E-5′-nucleotidase, CD73), and the nucleoside Ado (ADA) was employed to evaluate the alterations in purinergic system enzymes activities. Briefly, after protein adjustments of PBMCs and platelets, 20 μL of samples was added to the reaction mixture of each enzyme and preincubated at 37 °C for 10 min. The enzymatic reaction was initiated by adding the specific substrates for each enzyme: ATP and ADP for CD39 and AMP for CD73. After incubation at 37 °C for 70 min, the reactions were stopped by the addition of trichloroacetic acid (TCA, 10%), and the released inorganic phosphate due to ATP, ADP, and AMP hydrolysis was determined by using malachite green as the colorimetric reagent. A standard curve was prepared with KH2PO4. Controls were performed to correct for nonenzymatic hydrolysis. The absorbance was measured at 630 nm, and enzyme-specific activities were reported as nmol/Pi/min/mg of protein [41, 42]. For the ADA, activity was performed based on the measurement of ammonia produced when this enzyme acts in the excess of adenosine, following a previously published method [45]. In brief, 50 μL of cell suspension reacted with 21 mmol/L of adenosine (pH 6.5) at 37 °C for 60 min. After the incubation period, the reaction was stopped by the addition of 167.8 mM sodium nitroprusside, 106.2 mM phenol, and a sodium hypochlorite solution. Lastly, absorbance was read at 620 nm, and values were expressed as units/liter (U/L).
E-NTPDase-1 (CD39) and E-5′-NT (CD73) expression
Flow cytometry analysis was employed to investigate E-NTPDase-1 (CD39) and E-5'-NT (CD73) expression of CT subjects and COVID-19 patients. Monoclonal antibodies anti-CD45 (FITC), anti-CD39 (APC), and anti-CD73 (PE) for marking of the total leukocytes, E-NTPDase-1, and E-5′-NT, respectively, were used in the protocol. In brief, whole blood was initially collected with EDTA as an anticoagulant and used as a sample and analyzed by flow cytometry (BD Accuri C6) and analyzed by FlowJo V10 software.
Flow cytometry analysis of cytokines
The BD™ CBA Human Th1/Th2/Th17 Cytokine Kit was used to quantitatively measure interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-alpha (TNF-α), interleukin-17A (IL-17A), and interferon-gamma (IFN-γ) protein levels in a single sample. BD CBA assays provide a method of capturing a soluble analyte or set of analytes with known size and fluorescence beads, making it possible to detect analytes using flow cytometry.
Each capture bead was conjugated with a specific antibody. The detection reagent provided in the kit is a mixture of phycoerythrin (PE)-conjugated antibodies, which provides a fluorescent signal in proportion to the amount of bound analyte. When the capture beads and detector reagent are incubated with an unknown sample containing recognized analytes, sandwich complexes (capture bead + analyte + detection reagent) are formed. These complexes were measured using flow cytometry to identify particles with fluorescence characteristics of both the bead and the detector.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). The Shapiro–Wilk test tested normality. Outliers were excluded using the Grubbs test. About the study’s variables, the differences between COVID-19 patients and CT subjects were evaluated by unpaired Student’s t test or one-way ANOVA followed by the Tukey post hoc test. The results were presented as the mean and standard error. The differences in the probability of rejection of the null hypothesis as less than 5% (p < 0.05) were considered statistically significant.
Results
COVID-19 patients and control group clinical characteristics
As we presented in Table 1, in brief, a total of 111 subjects were included, 62 hospitalized patients with COVID-19, and 49 healthy controls. Of the 62 patients divided according to range severity, 40 were moderate (64.52%) and 22 severe (35.48%). All moderate cases were admitted in the infirmary, whereas in severe cases, 09 (40.91%) were admitted in the infirmary and 13 (59.09%) in ICU. About the gender, in moderate cases, 27 were female (67.50%) and 13 male (32.50%), whereas in severe cases, 14 (63.64%) were male and 08 (36.36%) female. Dyspnea, cough, fever, body weakness, myalgia, asthenia, diarrhea, airsickness and/or vomit, and odynophagia were the reported symptoms, being dyspnea and cough the most in both subgroups. The most frequent comorbidities were overweight in both subgroups (75% in moderate and 80% in severe cases).
SARS-CoV-2 infection alters purinergic enzyme activities in PBMCs and platelets
Enzymes of the purinergic system, such as E-NTPDase, 5′-NT, and ADA, have an essential role in alterations in the extracellular levels of signaling molecules. ATP and Ado are pivotal molecules and largely influence immune responses in peripheral and central tissues. Thus, the hydrolysis of nucleotides, including ATP, ADP, and AMP and the nucleoside Ado, was used to evaluate purinergic enzymes’ activities in PBMCs and platelets of COVID-19 patients and CT subjects (Figs. 2, 3 and 4). In PBMCs (Fig. 2A–C), ATP hydrolysis was decreased in COVID-19 patients compared to CT (p < 0.0001) (Fig. 2A), whereas AMP hydrolysis was increased in COVID-19 patients compared to controls (p < 0.001) (Fig. 2C). However, no statistical difference was observed for ADP hydrolysis comparing COVID-19 patients to controls (Fig. 2B). In platelets (Fig. 2D–F), the changes in ectoenzyme activities were more pronounced than in PBMCs, showing a significant increase in ATP (p < 0.01) (Fig. 2D), ADP (p < 0.001) (Fig. 2E), and AMP (p < 0.0001) (Fig. 2F) hydrolysis in COVID-19 patients compared to CT subjects.
ATP, ADP, and AMP hydrolysis in PBMCs and platelets of control subjects and COVID-19 patients. The E-NTPDase1 activity was measured by the hydrolysis of ATP and ADP, whereas the hydrolysis of AMP measured 5′-NT. Data are presented as the mean ± SEM. Statistical analysis: Student’s t test. Values with p < 0.05 were considered statistically significant. A A significant reduction in ATP hydrolysis in patients’ PBMCs is visible; B an increase in ADP hydrolysis is observed in PBMCs from COVID-19 patients; C AMP hydrolysis in PBMCs significantly higher in patients; D in platelets from COVID-19 patients, a significant increase in the hydrolysis of ATP, E ADP, and F AMP was observed. ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate differences from the control group
COVID-19 cases are often divided into moderate and severe, depending on the characteristics of patients, as severe cases can evolve to a more challenging clinical scenario with the need for ICU. Thus, to understand whether SARS-CoV-2 infection could trigger different responses in COVID-19 patients in distinct disease conditions, we investigated in Fig. 3 the impact of COVID-19 disease severity on ectoenzyme activities. According to the NIH and WHO guidelines, patients were grouped into moderate and severe cases and clinical conditions, together with disease outcomes (See Table 1). The results in PBMCs of moderate and severe COVID-19 patients (Fig. 3A–C), E-NTPDase1, and 5′-NT activities presented similar results to Fig. 2A–C, that is, ATP hydrolysis (Fig. 3A) was reduced in moderate and severe COVID-19 cases. In contrast, AMP hydrolysis (Fig. 3C) was increased in moderate and severe COVID-19 patients compared to the CT.
ATP, ADP, and AMP hydrolysis in PBMCs and platelets of control subjects and moderate and severe COVID-19 patients. The E-NTPDase activity was measured by the hydrolysis of ATP and ADP, whereas the hydrolysis of AMP measured 5′-NT. Data are presented as means ± SEM. When patients are divided on their severity, it is possible to highlight valuable findings. Thus, A shows a more significant reduction in ATP hydrolysis in PBMCs in moderate patients; B ADP hydrolysis levels in PBMCs are higher in severe patients; C as for AMP hydrolysis in PBMCs, it is also elevated in COVID-19 patients with higher values in severe cases. In the analysis of platelets, there is D an increase in ATP hydrolysis in critically ill patients, E a considerable increase in ADP in moderate and severe patients, still relating these two groups significantly, and F an increase in AMP hydrolysis in both groups significantly. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate differences between groups
Looking at the results regarding ectoenzyme activities of platelets from moderate and severe cases (Fig. 3D–F), ATP hydrolysis (Fig. 3D) was increased in severe cases compared to CT. ADP (Fig. 3E) and AMP (Fig. 3F) hydrolyses were found to be significantly increased in both groups (moderate and severe) compared to CT. Additionally, there was a significant increase in ADP hydrolysis (Fig. 3E) in severe cases compared to moderate cases.
Following the purinergic cascade, the activity of ADA was also measured by the breakdown of its substrate, Ado. Results showed that ADA activity was reduced in PBMCs of COVID-19 patients compared to CT (p = 0.03) (Fig. 4A). However, this was the opposite in platelets as COVID-19 presented increased hydrolysis of Ado compared to CT (p = 0.0053) (Fig. 4B). Furthermore, looking at the results of moderate and severe COVID-19 patients, we observed an increase in ADA activity only in platelets of moderate cases compared to CT (Fig. 4D). Regarding ADA activity in PBMCs, no significant differences were observed between groups (Fig. 4C).
ADA (adenosine deaminase—hydrolyzing adenosine) in platelets and in PBMCs of control (CT) and patients with COVID-19 (SARS-CoV-2). The assay is based on the direct measurement of ammonia produced by adenosine deaminase through adenosine breakdown. Results were expressed in units per liter (U/L). One unit (1 U) of ADA is defined as the amount of enzyme required to release 1 mmol of ammonia per minute from adenosine at standard assay conditions. Data are presented as mean ± SEM (standard error of the mean). ADA activity showed A a reduction in PBMCs from COVID-19 patients and B a significant increase in platelets. When divided into clinical severity, C moderate patients had a reduction in ADA from PBMCs, but without reaching statistical significance; and D in platelets, moderate patients had significantly increased ADA activity. Graphs A–B: Statistical analysis: Student’s t test. Graphs C–D: Statistical analysis: one-way ANOVA followed by Tukey post hoc test. Values with p < 0.05 were considered statistically significant. * indicates a significant difference from the control group (p < 0.05); ** indicates a significant difference from the control group (p < 0.01)
CD39 and CD73 expression is increased in total leukocytes of COVID-19 patients
Additionally, to ectonucleotidase activities, we analyzed CD39 and CD73 expression of COVID-19 patients and CT by flow cytometry to assess possible alterations (Fig. 5). Results showed a significant increase in CD39 (Fig. 5A) and CD73 (Fig. 5B) expression, as well as in the number of CD39 + (Fig. 5C) and CD73 + (Fig. 5D) cells from total leukocytes samples of COVID-19 patients when compared to CT. Furthermore, we also looked at differences in CD39 and CD73 expression comparing moderate and severe COVID-19 cases (Fig. 5E–H). Both patients’ groups, moderate and severe, presented an increased CD39 (Fig. 5E) and CD73 (Fig. 5F) expression compared to CT. However, only moderate COVID-19 cases presented increased numbers of CD39 + (Fig. 5G) and CD73 + (Fig. 5H) cells when compared to CT.
CD39 and CD73 expression in total leukocytes of control subjects and COVID-19 patients. Flow cytometry was employed to assess CD39 and CD73 expression (displayed as the median fluorescence intensity MFI) and the % of CD39 + and CD73 + cells. Data are presented as mean ± SEM (standard error of the mean). Representative flow cytometry histograms show gating with antibodies against CD45 (common leukocytes antigen) and staining with CD39 APC and CD73 PE (A). The enzymatic expression of CD39 (B) and CD73 (C) was significantly elevated in COVID-19 patients. The percentages of CD39 (D) and CD73 (E) on cells were also significantly increased in COVID-19 patients. In subgroups, both, moderated and severe, showed increased expression of CD39, being more significant in moderate patients (F); CD73 expression was significantly increased in both patient groups (G). The percentage of CD39 (H) and CD73 (I) on cells was significantly increased in moderate patients. Graphs B–E: Statistical analysis: Student’s t test. Graphs F–G: Statistical analysis: one-way ANOVA followed by Tukey post hoc test. Values with p < 0.05 were considered statistically significant. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate significant differences from the control group
Extracellular ATP levels
To confirm the alterations in levels of ATP extracellular, we measured by bioluminescence the levels of this nucleotide in the extracellular microenvironment, and these are present in Fig. 6. High levels of extracellular ATP were found in COVID-19 patients compared to CT. When we divide according to the severity levels, moderate cases express significant increase.
ATP extracellular levels in control subjects and COVID-19 patients. Extracellular ATP was determined as production of bioluminescence using a luciferin-luciferase reaction system (emission maximum ~ 560 nm at pH 7.8) through a commercial kit (Invitrogen®). The data was expressed in nM of ATP in serum. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate significant differences from the control group
SARS-CoV-2 infection impacts cytokines levels in COVID-19 patients
SARS-CoV-2 infection activates the immune system leading to a robust inflammatory response [46,47,48]. Thus, the levels of inflammatory mediators were analyzed in COVID-19 patients. Figures 7 and 8 display the levels of pro- and anti-inflammatory mediators investigated by flow cytometry from serum samples of COVID-19 patients and CT.
Cytokines levels in control subjects and COVID-19 patients. Flow cytometry was employed to assess the alterations in the concentration (pg/mL) of inflammatory markers in serum samples. Data are presented as mean ± SEM. In COVID-19 patients, when compared to controls, there is no difference between groups in IFN-y (A); a significant increase in IL-6 (B), IL-2 (C), IL-10 (D), and IL-17 (F); no difference between groups in TNF-a (E). Graphs A–F: Statistical analysis: Student’s t test. Values with p < 0.05 were considered statistically significant. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate significant differences from the control group
Cytokines levels in control subjects and moderate and severe COVID-19 patients. Flow cytometry was employed to assess the alterations in the concentration (pg/mL) of inflammatory markers in serum samples. Data are presented as mean ± SEM. A No difference between groups and subgroups in IFN-γ; B a significant increase in IL-6 in both patient groups, but higher in moderate subgroup; C a significant increase in IL-2 in severe patients than the control group; D a significant increase in IL-10 in both patient groups; E no significant difference between groups in TNF-a; F a significant increase in IL-17 in the severe cases of COVID-19. Graphs A–F: Statistical analysis: one-way ANOVA followed by Tukey post hoc test. Values with p < 0.05 were considered statistically significant. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001). All indicate significant differences from the control group
Figure 7A–F shows that COVID-19 patients presented increased IL-6, IL-2, IL-10, and IL-17 compared to CT. When looking at the results from moderate and severe COVID-19 cases (Fig. 8A–F), IL-6 (Fig. 8B) and IL-10 (Fig. 8D) levels were significantly increased in both moderate and severe COVID-19 cases when compared to CT. However, IL-2 (Fig. 8C) and IL-17 (Fig. 8F) were significantly increased only in severe COVID-19 cases compared to CT.
Discussion
There has been substantial research devoted to understanding the pathophysiology of SARS-CoV-2 infection and COVID-19. However, cellular signaling pathways, closely associated with the immune system that may drive disease progression and outcome, have not been assessed yet. Here, for the first time, we provide robust evidence that COVID-19 patients present alterations in purinergic signaling and immune response, and both these seem to be correlated. This data corroborates previous studies’ hypothesis, which suggested a possible link between SARS-CoV-2 infection and purinergic signaling [32,33,34,35, 49].
In this context, inflammation has been recognized as a hallmark in COVID-19, and evidence supports that the magnitude of inflammatory responses and CS are directly related to the severity of the disease [50]. Thus, COVID-19 patients were divided into moderate and severe cases according to the clinical characteristics of patients and NIH [38] and WHO [39] guidelines to elucidate a possible linkage between the changes observed in purinergic system components with the immune system alterations and the damage caused by COVID-19. From this, we found significant alterations in purinergic signaling, specifically in ectoenzyme activities both in PBMCs and platelets, displayed by the differences in the levels of signaling molecules, in the expression of CD39 and CD73 of total leukocytes, and in levels of extracellular ATP and cytokines of COVID-19 patients (see Figs. 2, 3, 4, 5,6, 7 and 8).
Therefore, one of the relevant aspects of our study is the relationship between disease severity and patient gender. According to our statistical data, men are more likely to develop a severe form of COVID-19, as in other studies, confirming this finding, since, analyzing 239,709 patients in Italy, the lethality was 17.7% in men and 10.8% in women, with 59% of all deaths being men [51]. Data from the WHO and Chinese scientists show that in all cases, 2.8% of men infected with SARS-CoV-2 have a fatal outcome, compared to about 1.7% of women. Additionally, data from hospitals in Hong Kong state that 32% of male patients with COVID-19 need intensive care or have died, while 15% of female patients need this demand [52].
There are hypotheses in the literature about a greater susceptibility to infection related to gender or determinants related to sex that can worsen prognosis. On the one hand, estrogen, the inactivation of the X chromosome or reduced methylation, may increase the expression of ACE2 in women, balancing the physiology of the renin-angiotensin system (RAS) regulatory axis by the availability of ACE2. Furthermore, low androgen levels in women can regulate low levels of TMPRSS2 expression. Considering the dependence of both structures for SARS-CoV-2 infection is understood how these changes are protective [51].
Older age, male gender, comorbidities such as cardiovascular disease or hypertension, and diabetes and obesity were identified as risk factors for more severe COVID-19 [53, 54]. Furthermore, as COVID-19 has been closely associated with thrombotic events, clinical evidence demonstrates that cardiovascular events are more prevalent in men than women [55, 56] and greater reactivity platelet count in men than in women [57]. Women have higher CD3 + cells than men, closely associated with the results, mainly deviating from the more significant helper T cells [58]. The positive influence of the female gender on the level of CD4 + cells in human peripheral blood is well known [59, 60].
But, the more important point of this study is the purinergic signaling in COVID-19, which has not been evidenced until now. Thus, considering that the nucleotides and nucleosides constitute paramount signaling molecules in purinergic-mediated reactions [61], we looked for the outcomes of this molecule’s hydrolysis of COVID-19 patients and detected that there was a significant decrease in ATP (Fig. 2A) and an increase in AMP (Fig. 2C) hydrolysis in PBMCs. In platelets, the hydrolysis of ATP, ADP, and AMP nucleotides was increased compared to the CT (Fig. 2D–F).
In the subgroups, we identified that the reduced ATP hydrolysis in PBMCs was considerably higher in moderate patients (Fig. 3A). In contrast, patients with severe cases had higher levels of AMP hydrolysis in PBMCs than in moderate ones (Fig. 3C). Moderate patients presented ATP increased (Fig. 3A), which could be responsible for a possible disease progression and AMP hydrolysis in critical cases to increase Ado levels and reduce exacerbated inflammation [62]. Furthermore, the AMP consumption increases the Ado product, which acts in an immunosuppressive manner [63]. However, reducing ATP hydrolysis results in greater nucleotide availability, which is associated with a proinflammatory environment. On the other hand, in platelets, when comparing moderate and severe COVID-19 patients, the most significant expression of these numbers was in the severe subgroup. Although the ATP hydrolysis was not significant in moderate patients, ADP and AMP hydrolysis in platelets showed significant results in both subgroups of patients (Fig. 3D–F). This result suggests that the body regulates thrombus formation, given the activity of AMP and Ado on platelet aggregation. Furthermore, evidence indicates more reactive platelets in patients with COVID-19, contributing to the different reports of thrombotic risk in the disease [64,65,66].
Following the above data, in platelet activation, ADP acts on the recruitment and activation of platelets, while ATP can activate polymorphonuclear cells (PMN) [67]. With the hydrolysis of these nucleotides, AMP controls the microthrombus formation and oxidative stress in the inflammatory microenvironment. Finally, the end product of the enzymatic activity, Ado, is responsible for platelet inhibition by reducing free radicals and acting as a vasodilator in microvascular beds [68].
Since the detection that the nucleotides and nucleosides hydrolysis have been altered, we followed by searching whether the expression of CD39 and CD73 also was changed and, as presumed, both were significantly higher in total leukocytes of patients with COVID-19 compared to the controls. When separated by the severity of the condition, patients with moderate clinical features had higher levels (Fig. 5A–H). Few papers had demonstrated the expression of these ectoenzymes in COVID-19, including Ahmadi et al. [69] that showed no differences in the expression of CD39 and CD73 in lymphocytes in COVID-19 patients. It is important to highlight that here results presented, by the alterations in ectoenzyme activities and expression, suggest that purinergic signaling is strongly related with physiopathology in SARS-CoV-2 infection and consequently in the COVID-19.
Considering other disease contexts, some papers have shown increased activity and expression of CD39 on lymphocytes from individuals infected with the human immunodeficiency virus (HIV), in Tregs cells expression of HIV-infected individuals, and in cytomegalovirus infection [70,71,72,73]. This fact seems to be connected with the role of immune response in the virus infections to regulation, such as SARS-CoV-2. The CD39-CD73 axis hydrolytic action on purinergic cascade has been shown as an essential immunoregulatory effect, given the Ado production by a breakdown of the ATP to pre-anti-inflammatory molecules, the ADP and AMP [74].
ADA, an end enzyme of purinergic cascade responsible for the hydrolysis of Ado into inosine, is closely associated with improvements in inflammatory conditions and ARDS present in severe cases of COVID-19 [75]. In view of this point, we looked for ADA activity alterations and detected increased activity in platelets and a decrease in PBMCs compared to the control. The moderate subgroup of platelets had increased in activity, whereas in other subgroups, no statistical significance was observed in COVID-19 patients (Fig. 4A–D). Ado plays a crucial role in regulating the interaction between lymphocytes and controlling lymphocyte traffic in response to tissue injury or infection. Thus, a reduction in ADA activity can provide higher levels of Ado [70]. From this perspective, considering the high lymphocyte activity during the infection, this reduction could be associated with an attempt at immune regulation. Thereby, the nucleoside could act not only on immune cells but also on the T cell receptor (TCR) since, by activating adenosine receptors, it can modulate its activity [76].
Otherwise, evidence indicates that ADA could compete with SARS-CoV-2 for binding to the CD26 receptor [76], acting in a non-enzymatic way in physical protection against viral infection. Its expression in platelets is closely associated with Ado consumption resulting from increased nucleotide hydrolysis by ADA. Although Ado is associated with reduced platelet activation, decreased thrombosis, and attenuation of inflammation [76], this increased activity is understandable due to the high levels of hydrolyzed nucleotides.
Considering the all find as mentioned above and the importance of ectoenzymes in purinergic signaling, due to potential hydrolysis of ATP to Ado, which may attenuate the inflammatory process [77], few studies have reported that the role of ectonucleotidases in COVID-19 and, specifically, knowledge about these enzymes in PBMCs and platelets is scarce. Thus, it is essential to highlight that we are the first research team to show that significantly ectonucleotidase activities and expression are altered in PBMCs and platelets of patients with COVID-19.
In addition to all previous admeasurement purinergic-related signaling, we also quantify the ATP extracellular levels. This approach is justified from ATP can function as a DAMP [69, 78], and the activation of purinergic receptors, notably P2X7, can trigger a cascade of reactions that culminate in the release of inflammatory cytokines and cellular apoptosis [79, 80]. Furthermore, the excessive permanence of ATP in the outside environment may generate an uncontrolled activation of the immune system [81], triggering a CS, characterized by the exacerbated release of inflammatory mediators, including IL-1β, GMCSF, IFN-y, IL-10, IL-2, IL-6, IL-17, and TNF-α [82]. Interestingly, we detected high levels of extracellular ATP in COVID-19 patients compared to CT, and moderate was the subgroup that expressed significant increases (Fig. 6).
Upon cellular damage caused by infection processes such as COVID-19 that recruit the immune system and may generate extensive inflammatory reactions, intracellular ATP may be released to the extracellular milieu, mainly through cell lysis or cell channels such as pannexin-1, operating as a signal activating purinergic receptors, such as the P2X7, expressed on immune cells such as neutrophils, eosinophils, monocytes, macrophages, mast cells, and lymphocytes [81]. It is important to highlight that pannexin-1 is involved in amplifications of P2X7 activation due to the release amount of ATP in the extracellular environment in response to viral infections [83].
In view of pleiotropic actions of extracellular, an interesting paper recently published by Abraham et al. [84] showed that oral ATP supplementation may benefit general patients with COVID-19. Evidence indicated that cystic fibrosis (CF) patients have elevated blood and extracellular ATP, due to release through the pannexin-1 membrane bound protein in both RBCs and epithelial cells that is modulated by cystic fibrosis transmembrane conductance regulator (CFTR). Several studies have demonstrated that patients with CF, although somewhat more susceptible to SARS-CoV-2 infection, demonstrate a survival rates in the face of infection. These findings served as clues for testing the effects of ATP supplementation for elderly SARS CoV-2 infected individuals. Increasing ATP levels in these age ATP depleted individuals is postulated to promote an effective immune response after infection with SARS-CoV-2. The depleted ATP levels correlated with increasing an aging in the general population is associated with diminished immune responses, as well as causing muscle fatigue and breathing problems. These hypotheses were tested in a pilot clinical trial. Elderly COVID-19-positive individuals taking between 1.2 and 1.6 g of oral ATP daily had an improved COVID-19 survival compared to comparable elderly individuals without ATP supplementation. It is suggested that there is micro clot attenuation and tissue regeneration achieved by mimicking ATP-metabolism similar to that observed in CF individuals. The authors recommended that elderly individuals exposed to COVID-19 should be started on low-dose ATP consumption. Full-dose ATP should be administered to elderly individuals testing positive for COVID-19. Early ATP supplementation in positive COVID-19 cases led to rapid clearance of the SARS-CoV-2. ATP supplementation should be reduced and discontinued within 1 to 2 months of the viral clearance. With prolonged ATP supplementation in the elderly, there is risk of systemic uric acid buildup of and gout.
One of the characteristics of critically ill patients with COVID-19 is the increase in proinflammatory cytokines such as IL-6, TNF, IL-1, IL-2, IL-17, IFN-γ, G-CSF, MCP-1, and IP-10. These seem to be higher in critically ill patients who still have low T, B cells and natural killer (NK), further aggravating ARDS [85]. In addition, Shi et al. [86] observed an increase in IL-10 and IL-6 in critically COVID-19 patients with pneumonia. Thus, due to the literature hypotheses about the ATP-CS axis, as presented above, finally, we looked for this linkage between the purinergic signaling and CS from an immune response. Thus, we confirmed in our study a considerable increase in IL-6, IL-17, IL-10, and IL-2, whereas no statistical significance was observed for IFN-γ and TNF-α when comparing COVID-19 patients to the controls. Still, we divided the COVID-19 patients for severity, being that both moderate and severe patients showed IL-6 (Figs. 6B and 7B), IL-17 (Figs. 6F and 7F), IL-10 (Figs. 6D and 7D), and IL-2 (Figs. 6D and 7D) with statistically significant results compared to controls. In the same way, IFN-γ (Figs. 6A and 7A) and TNF-α (Figs. 6E and 7E) have no significance when divided into subgroups.
The IL-6, an important proinflammatory mediator, plays a central role in inducing the acute phase response [87], being that different studies relate IL-6 levels with early cases of lung injury and predictive factors for prolonged mechanical ventilation, organ dysfunction, morbidity, and mortality in lung diseases [88, 89]. Besides, there may be a relationship between IL-6 levels and SARS-CoV-2 infection, since the structural protein N of the coronaviruses, which acts directly on the entry of the virus into the host cell, works by increasing the expression of IL-6 in human airway epithelial cells [90]. Thus, our results showed high levels of IL-6, however, more significant in moderate patients and a considerable reduction in severe patients. These results may be related to the viral induction itself, by the action of other cytokines on IL-6, or even an organic attempt to reduce exacerbated inflammation.
Together with IL-6 stimulation itself, the extracellular ATP released during T cell activation can increase IL-2 production [91], confirming the values detected in our study. Thus, IL-2, known as the T cell growth factor, is produced mainly by activated CD4 + T cells and CD8 + 18—21 T cells. Therefore, high concentrations of this cytokine are responsible for activating CD4 T cells + and CD8 + . On the other hand, low concentrations are responsible for inhibiting these cells by maintaining the activity and survival of Treg cells [92]. Our results showed an increase in IL-2 in severe cases when compared to moderate ones. The cytokine specificity can explain this, and its high levels in patients with COVID-19 may indicate T cell activation, which occurs more significantly in critical cases.
Interestingly, in this context, a research team explored the regulatory effects of adenosine on inflammatory mediators. Through a single-center case–control study of 24 enrolled subjects with SARS-CoV-2 infections confirmed by RT-PCR, researchers applied a new off-label therapeutic treatment using deliberately inhaled adenosine (Krenosin®) in COVID-19 patients parallel to another standard therapeutic protocol, with the aim of reducing inflammation, the appearance of CS, and, therefore, improving the prognosis. The dosage used was 9 mg every 12 h for the first 24 h, and, subsequently, every 24 h for 5 days. Surprisingly, there has been, among others, a reduction in the severity of inflammation and the time for the occurrence of lung injuries [93]. These data once again confirm the involvement of purinergic signaling in the pathophysiology of COVID-19 and as a promising therapeutic target.
Another cytokine with high levels is IL-17, producing proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α [94]. These values stand out in severe patients and may indicate an exacerbation of the CS and a worsening of the prognosis, as it acts on the recruitment of neutrophils to the lungs and organ dysfunction in ARDS. Furthermore, these levels are directly associated with IL-6 activity, a fact confirmed by Hou et al. [95], who found in murine viral models that the excessive level of IL-6 promotes the generation of Th17 cells. These cells release IL-6 and IL-17, cytokines that synergistically promote viral persistence, protecting virus-infected cells from apoptosis.
In contrast, a molecule produced mainly by CD4 + T cells, which is a master regulator of immunity to infection, is the anti-inflammatory cytokine IL-10 [96]. However, recent findings indicate that a high level of exogenous IL-10 promotes cytotoxicity of CD8 + T cells and an intermediate level of endogenous IL-10 induces depletion of CD8 + T cells in the tumor [97]. In addition, IL-10 can inhibit IL-2 secretion, which could explain the reduction in IL-2 in moderate patients, associated with higher IL-10 values. Gong et al. [98] demonstrated that although IL-2R and IL-10 levels were associated with disease severity, they mainly contributed to the inhibition of the inflammatory response. Consequently, this hypothesis further developed the idea that it might suggest the simultaneous inflammatory and anti-inflammatory reaction.
The maximum antiviral activity of T cells during acute infection coincides with the maximum anti-inflammatory expression of IL-10, suggesting that a super aggressive adaptive immune response is already counterbalanced during the viral clearance phase and does not necessarily arise as a result of tissue damage. Confirming this hypothesis, we observed that IL-10 is elevated in both groups of patients with COVID-19, as shown in another study [99]. However, some previous studies with viral infections have increased immunosuppressive molecules as a tactic of the virus itself [100]. Thus, the increase in the local production of adenosine, associated with the upstream of ectonucleotidases, generates an immunosuppressive and antithrombotic microenvironment, which allows the virus to enter the target cell [62] more easily.
Considering pandemic status, several research teams have spared no effort to look for new strategies to mitigate this situation, and purinergic modulation is widely highlighted in the literature for its therapeutic potential in this context. Thus, we found significant findings that confirm the alterations in the purinergic signaling and immune response in COVID-19, and both seem to be correlated. Besides, it became more evident that the range of severity directly impacts these alterations. Our study supports the previous hypothesis that the purinergic system may target SARS-CoV-2 and future disease treatments. Finally, we suggest that new studies must be developed to complement and ensure the purinergic pathway clinical application effectively for COVID-19.
Data and materials availability
All data are available in the main text or supplementary materials.
References
Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M et al (2020) The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. https://doi.org/10.1007/s11481-020-09944-5
WHO. World Health Organization (2021) WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 29 Aug 2021
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Hartung H-P, Aktas O (2020) COVID-19 and management of neuroimmunological disorders. Nat Rev Neurol 16:347–348. https://doi.org/10.1038/s41582-020-0368-9
Berlin DA, Gulick RM, Martinez FJ (2020) Severe COVID-19. N Engl J Med. https://doi.org/10.1056/NEJMcp2009575
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:934–943. https://doi.org/10.1001/jamainternmed.2020.0994
Rouse BT, Sehrawat S (2010) Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 10:514–526. https://doi.org/10.1038/nri2802
Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, Tan K-S, Wang D-Y, Yan Y (2020) The origin, transmission, and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res 7(1):11. https://doi.org/10.1186/s40779-020-00240-0
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/s0140-6736(20)30183-5
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L et al (2020) Pathological findings of COVID19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation, and intervention. Nat Rev Immunol 20:363–374. https://doi.org/10.1038/s41577-020-0311-8
Ye Q, Wang B, Mao J (2020) The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect 80:607–613. https://doi.org/10.1016/j.jinf.202003.037
Burnstock G, Knight GE (2004) Cellular distribution and function of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304. https://doi.org/10.1016/S0074-7696(04)40002-3
Atkinson B, Dwyer K, Enjyoji K, Robson SC (2006) Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36:217–222. https://doi.org/10.1016/j.bcmd.2005.12.025
Burnstock G, Verkhratsky A (2012) Purinergic signaling, and the nervous system, Springer. ISBN:3642288626. https://doi.org/10.1007/978-3-642-28863-0
Visovatti SH, Hyman MC, Bouis D, Neubig R, McLaughlin VV, Pinsky DJ (2012) Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PLoS One 7:408–429. https://doi.org/10.1371/journal.pone.0040829
Bagatini MD, dos Santos AA, Cardoso AM, Mânica A, Reschke CR, Carvalho FB (2018) The impact of purinergic system enzymes on noncommunicable, neurological, and degenerative diseases. J Immunol Res 2018:1–21. https://doi.org/10.1155/2018/4892473
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ectonucleotidases. Purinergic Signalling 8:437–502. https://doi.org/10.1007/s11302-012-9309-4
Yegutkin GG (2008) Nucleotide-and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta Mol Cell Res 1783:673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024
Schetinger MRC, Morsch VM, Bonan CD, Wyse ATS (2007) NTPDase and 5-nucleotidase activities in physiological and disease conditions: new perspectives for human health. BioFactors 31:77–98. https://doi.org/10.1002/biof.5520310205
Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis Found Symp 276:113–128 PMID: 16805426
Zanini D, Schmatz R, Pelinson LP, Pimentel VC, da Costa P, Cardoso AM, Martins CC, Schetinger CC, Baldissareli J, do Carmo Araújo M et al (2013) Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancer. Mol Cell Biochem 374:137–148. https://doi.org/10.1007/s11010-012-1513-6
Ledderose C, Woehrle T, Ledderose S, Strasser K, Seist R, Bao Y, Zhang J, Junger WG (2016) Cutting off the power: inhibition of leukemia cell growth by pausing basal ATP release and P2X receptor signaling? Purinergic Signaling 12:439–451. https://doi.org/10.1007/s11302-016-9510-y
Hu L-P, Zhang X-X, Jiang S-H, Tao L-Y, Li Q, Zhu L-L, Yang M-W, Huo Y-M, Jiang Y-S, Tian G-A et al (2019) Targeting purinergic receptor P2Y2 prevents the growth of pancreatic ductal adenocarcinoma by inhibiting cancer cell glycolysis. Clin Cancer Res 25:1318–1330. https://doi.org/10.1158/1078-0432.CCR-18-2297
Hevia MJ, Castro P, Pinto K, Reyna-Jeldes M, Rodríguez-Tirado F, Robles-Planells C, Ramírez-Rivera S, Madariaga JA, Gutierrez F, López J et al (2019) Differential effects of purinergic signaling in gastric cancer-derived cells through P2Y and P2X receptors. Front Pharmacol 10:612. https://doi.org/10.3389/fphar.2019.00612
Mânica A, Bonadiman BDSR, Cardoso AM, Paiz A, Siepko C, de Souza JVG, Moreno M, Moreno A, Schetinger MRC, Morsch VM et al (2019) The signaling effects of ATP on melanoma-like skin cancer. Cell Signal 59:122–130. https://doi.org/10.1016/j.cellsig.2019.03.021
Zanini D, Manfredi LH, Pelinson LP, Pimentel VC, Cardoso AM, Carmo Araújo Gonçalves VD, Dos Santos CB, Gutierres JM, Morsch VM, Leal DBR et al (2019) ADA activity is decreased in lymphocytes from patients with advanced stage of lung cancer. Med Oncol 36:78. https://doi.org/10.1007/s12032-019-1301-1
Mosher KI, Wyss-Coray T (2014) Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol 88:594–604. https://doi.org/10.1016/j.bcp.2014.01.008
Freire D, Reyes RE, Baghram A, Davies DL, Asatryan L (2019) P2X7 receptor antagonist A804598 inhibits inflammation in brain and liver in C57BL/6J mice exposed to chronic ethanol and high fat diet. J Neuroimmune Pharmacol 14:263–277. https://doi.org/10.1007/s11481-018-9816-3
Reichert KP, Castro MFV, Assmann CE, Bottari NB, Miron VV, Cardoso A, Stefanello N, Morsch VMM, Schetinger MRC (2021) Diabetes and hypertension: pivotal involvement of purinergic signaling. Biomed Pharmacother 137:111273. https://doi.org/10.1016/j.biopha.2021.111273
Burnstock G, Novak I (2013) Purinergic signalling and diabetes. Purinergic Signalling 9:307–324. https://doi.org/10.1007/s11302-013-9359-2
Dos Anjos F, Simões JLB, Assmann CE, Carvalho FB, Bagatini MD (2020) Potential therapeutic role of purinergic receptors in cardiovascular disease mediated by SARS-CoV-2. J Immunol Res 2020:1–14. https://doi.org/10.1155/2020/8632048
Di Virgilio F, Tang Y, Sarti AC, Rossato M. (2020)A rationale for targeting the P2X7 receptor in Coronavirus disease 19. Br J Pharmacol 177(21):4990–4. https://doi.org/10.1111/bph.15138
Pacheco PAF, Faria RX (2021) The potential involvement of P2X7 receptor in COVID-19 pathogenesis: a new therapeutic target? Scand J Immunol. https://doi.org/10.1111/sji.12960
Ribeiro DE, Oliveira-Giacomelli A, Glaser T, Arnaud-Sampaio VF, Andrejew R, Dieckmann L, Baranova J, Lameu C, Ratajczak MZ, Ulrich H (2021) Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol Psychiatry 26(4):1044–1059. https://doi.org/10.1038/s41380-020-00965-3
Di Virgilio F, Sarti AC, Coutinho-Silva R (2020) Purinergic signalling, DAMPs and inflammation. Am J Physiol Cell Physiol 318:C832–C835. https://doi.org/10.1152/ajpcell.00053.2020
Franciosi MLM, Lima MDM, Schetinger MRC, Cardoso AM (2021) Possible role of purinergic signaling in COVID-19. Mol Cell Biochem. https://doi.org/10.1007/s11010-021-04130-4
NHI. National Institutes of Health. COVID-19 treatment guidelines panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 29 Aug 2022
WHO. World Health Organization (2021). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. 13 March 2020. Reference numver: WHO/2019-nCoV/Clinical/2020.1
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest 97:77–89 PMID: 4179068
Pilla C, Emanuelli T, Frasetto SS, Battastini AM, Dias RD, Sarkis JJ (1996) ATP diphosphohydrolase activity (apyrase, EC 3.6.1.5) in human blood platelets. Platelets 7:225–230. https://doi.org/10.3109/09537109609023582
Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzantti CM et al (2003) Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res 109:189–194. https://doi.org/10.1016/s0049-3848(03)00178-6
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:218–254. https://doi.org/10.1006/abio.1976.9999
Karamohamed S, Guidotti G (2001) Bioluminometric method for real-time detection of ATPase activity. Biotechniques 31:420–425
Giusti G, Galanti B (1984) Colorimetric method. HU Berg-meyer, Methods of Enzymatic Analysis, 3rd edn. Verlag Chemie, Weinheim, pp 315–323
Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C (2020) Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther
De Cken M, Dhaliwal K, Danielsen AC, Gautron AS, Dominguez-Villar M (2019) TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal. 12:eaaw1347. https://doi.org/10.1126/scisignal.aaw1347
Fung TS, Liu DX (2019) Human coronavirus: host-pathogen interaction. Annu Rev Microbiol 73:529–557. https://doi.org/10.1146/annurev-micro-020518-115759
Simões JLB, Bagatini MD (2021) Purinergic signaling of ATP in COVID-19 associated Guillain-Barré syndrome. J Neuroimmune Pharmacol 16(1):48–58. https://doi.org/10.1007/s11481-020-09980-1. Epub 2021 Jan 18. PMID: 33462776; PMCID: PMC7813171
Wang J, Jiang M, Chen X, Montaner LJ (2020) Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108(1):17–41. https://doi.org/10.1002/JLB.3COVR0520-272R. Epub 2020 Jun 13. PMID: 32534467; PMCID: PMC7323250
Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V (2020) COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males?. Int J Mol Sci 21(10):3474. https://doi.org/10.3390/ijms21103474. PMID: 32423094; PMCID: PMC7278991
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627
Nakeshbandi M, Maini R, Daniel P, Rosengarten S, Parmar P, Wilson C, Kim JM, Oommen A, Mecklenburg M, Salvani J et al (2020) The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes (Lond) 44(9):1832–1837. https://doi.org/10.1038/s41366-020-0648-x. Epub 2020 Jul 25. PMID: 32712623; PMCID: PMC7382318
Mahmood SS, Levy D, Vasan RS, Wang TJ (2014) The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383(9921):999–1008. https://doi.org/10.1016/S0140-6736(13)61752-3. Epub 2013 Sep 29. PMID: 24084292; PMCID: PMC4159698
Di Giosia P, Passacquale G, Petrarca M, Giorgini P, Marra AM, Ferro A (2017) Gender differences in cardiovascular prophylaxis: Focus on antiplatelet treatment. Pharmacol Res 119:36–47. https://doi.org/10.1016/j.phrs.2017.01.025 Epub 2017 Jan 25 PMID: 28131875
Wiśniewski A, Sikora J, Filipska K, Kozera G (2019) Assessment of the relationship between platelet reactivity, vascular risk factors and gender in cerebral ischaemia patients. Neurol Neurochir Pol 53(4):258–264. https://doi.org/10.5603/PJNNS.a2019.0028 Epub 2019 Jul 25 PMID: 31343071
Löhr P, Schiele S, Arndt TT, Grützner S, Claus R, Römmele C et al (2021) Impact of age and gender on lymphocyte subset counts in patients with COVID-19. Cytometry. https://doi.org/10.1002/cyto.a.24470
Kverneland AH, Streitz M, Geissler E, Hutchinson J, Vogt K, Boës D, Niemann N, Pedersen AE, Schlickeiser S, Sawitzki B (2016) Age and gender leukocytes variances and references values generated using the standardized ONE-Study protocol. Cytometry A 89(6):543–564. https://doi.org/10.1002/cyto.a.22855 Epub 2016 May 3 PMID: 27144459
Andreu-Ballester JC, García-Ballesteros C, Benet-Campos C, Amigó V, Almela-Quilis A, Mayans J, Ballester F (2012) Values for αβ and γδ T-lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: assessment by age and gender. Cytometry B Clin Cytom 82(4):238–244. https://doi.org/10.1002/cyto.b.21020 Epub 2012 Apr 26 PMID: 22539222
Zhao Z, Xie J, Yin M, Yang Y, He H, Jin T, Li W, Zhu X, Xu J, Zhao C et al (2020) Clinical and laboratory profiles of 75 hospitalized patients with novel coronavirus disease 2019 in Hefei, China. MedRxiv. https://doi.org/10.1101/2020.03.01.20029785
Burnstock G (2018) Purine and purinergic receptors. Brain Neurosci Adv 2:1–10. https://doi.org/10.1177/2398212818817494
Antonioli L, Pacher P, Vizi ES, Haskó G (2013) CD39 and CD73 in immunity and inflammation. Trends Mol Med 19(6):355–67. https://doi.org/10.1016/j.molmed.2013.03.005. Epub 2013 Apr 17. PMID: 23601906; PMCID: PMC3674206
Takenaka MC, Robson S, Quintana FJ (2016) Regulation of the T cell response by CD39. Trends Immunol 37(7):427–439. https://doi.org/10.1016/j.it.2016.04.009. Epub 2016 May 25. PMID: 27236363; PMCID: PMC5215082
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F et al (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x. Epub 2020 May 4. PMID: 32367170; PMCID: PMC7197634
Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J et al (2020). Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18(8):1995–2002. https://doi.org/10.1111/jth.14888. Epub 2020 Jul 27. PMID: 32369666; PMCID: PMC7497052
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV et al (2020) Confirmação da alta incidência cumulativa de complicações trombóticas em pacientes criticamente enfermos de UTI com COVID-19: uma análise atualizada. Thromb Res 191:148–150. https://doi.org/10.1016/j.thromres.2020.04.041. Epub 2020, 30 de abril. PMID: 32381264; PMCID: PMC7192101
Wolska N, Rozalski M (2019) Blood platelet adenosine receptors as potential targets for anti-platelet therapy. Int J Mol Sci 20(21):5475. https://doi.org/10.3390/ijms20215475.PMID:31684173;PMCID:PMC6862090
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med 180(7):934–943. https://doi.org/10.1001/jamainternmed.2020.0994. Erratum. In: JAMA Intern Med 2020 Jul1; 180(7):1031. PMID:32167524; PMCID: PMC7070509
Ahmadi P, Hartjen P, Kohsar M, Kummer S, Schmiedel S, Bockmann JH, Fathi A, Huber S, Haag F, Schulze Zur Wiesch J (2020) Defining the CD39/CD73 axis in SARS-CoV-2 infection: the CD73- phenotype identifies polyfunctional cytotoxic lymphocytes. Cells 9(8):1750. https://doi.org/10.3390/cells9081750. PMID: 32707842; PMCID: PMC7464076
Song JW, Huang HH, Zhang C, Yang HG, Zhang JY, Xu RN, Jin L, Shi M, Wang FS, Jiao YM (2019) Expression of CD39 is correlated with HIV DNA levels in naïve Tregs in chronically infected ART Naïve patients. Front Immunol 17(10):2465. https://doi.org/10.3389/fimmu.2019.02465.PMID:31681335;PMCID:PMC6811520
Schulze Zur Wiesch J, Thomssen A, Hartjen P, Tóth I, Lehmann C, Meyer-Olson D, Colberg K, Frerk S, Babikir D, Schmiedel S et al (2011) Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol 85(3):1287–97. https://doi.org/10.1128/JVI.01758-10. Epub 2010 Nov 3. PMID: 21047964; PMCID: PMC3020516
Mora-García M, López-Cisneros S, Gutiérrez-Serrano V, García-Rocha R, Weiss-Steider B, Hernández-Montes J, Sánchez-Peña HI, Ávila-Ibarra LR, Don-López CA, Muñóz-Godínez R et al (2019) HPV-16 Infection Is associated with a high content of CD39 and CD73 ectonucleotidases in cervical samples from patients with CIN-1. Mediators Inflamm 7:4651627. https://doi.org/10.1155/2019/4651627
Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, Wedderburn L (2010) High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol 185:134–143
Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25high-FOXP3+ regulatory T cells. J Biol Chem 285:7176–7186. https://doi.org/10.1074/jbc.M109.047423
Geiger JD, Khan N, Murugan M, Boison D (2020) Possible role of adenosine in COVID-19 pathogenesis and therapeutic opportunities. Front Pharmacol 26(11):594487. https://doi.org/10.3389/fphar.2020.594487. PMID:33324223;PMCID:PMC7726428
Linden J, Cekic C (2012) Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol 32(9):2097–103. https://doi.org/10.1161/ATVBAHA.111.226837. Epub 2012 Jul 5. PMID: 22772752; PMCID: PMC4476649
Foresta C, Rocca MS, Di Nisio A (2021) Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest 44(5):951–956. https://doi.org/10.1007/s40618-020-01383-6. Epub 2020 Sep 16. PMID: 32936429; PMCID: PMC7492232
Wang W, Hu D, Feng Y, Wu C, Song Y, Liu W, Li A, Wang Y, Chen K, Tian M et al (2020) Paxillin mediates ATP-induced activation of P2X7 receptor and NLRP3 inflammasome. BMC Biol 18(1):182. https://doi.org/10.1186/s12915-020-00918-w.PMID:33243234;PMCID:PMC7694937
Burnstock G, Boeynaems JM (2014) Purinergic signalling and immune cells. Purinergic Signal 10:529–564. https://doi.org/10.1007/s11302-014-9427-2
Burnstock G (2016) P2X ion channel receptors and inflammation. Purinergic Signalling 12:59–67. https://doi.org/10.1007/s11302-015-9493-0
Burnstock G, Knight GE (2018) The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 14:1–18. https://doi.org/10.1007/s11302-017-9593-0
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes immunusuppression. J Lancet 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0. PMID: 32192578; PMCID: PMC7270045
Zhang C, He H, Wang L, Zhang N, Huang H, Xiong Q et al (2017) Virus-triggered ATP release limits viral replication through facilitating IFN-β production in a P2X7-dependent manner. J Immunol 199:1372–1381. https://doi.org/10.4049/jimmunol.1700187
Abraham EH, Guidotti G, Rapaport E, Bower D, Brown J, Griffin RJ, Donnelly A, Waitzkin ED, Qamar K, Thompson MA et al (2021) Cystic fibrosis improves COVID-19 survival and provides clues for treatment of SARS-CoV-2. Purinergic Signalling 17:399–410. https://doi.org/10.1007/s11302-021-09771-0
Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, Zhang Z, Qin Y, Li X, Zhao D et al (2020) Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev 7:1003–1011. https://doi.org/10.1093/nsr/nwaa037
Shi H, Wang W, Yin J, Ouyang Y, Pang L, Feng Y, Qiao L, Guo X, Shi H, Jin R et al (2020) The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis 11:429. https://doi.org/10.1038/s41419-020-2636-4
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295. https://doi.org/10.1101/cshperspect.a016295. PMID:25190079;PMCID:PMC4176007
Butt Y, Kurdowska A, Allen TC (2016) Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 140(4):345–350. https://doi.org/10.5858/arpa.2015-0519-RA. PMID: 27028393
Chepurnova DA, Samoilova EV, Anisimov AA, Verin AD, Korotaeva AA (2018) Compounds of IL-6 receptor complex during acute lung injury. Bull Exp Biol Med 164(5):609–611. https://doi.org/10.1007/s10517-018-4042-9. Epub 2018 Mar 26. PMID: 29577202; PMCID: PMC6428418
Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA (2020) The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol 146(3):518-534.e1. https://doi.org/10.1016/j.jaci.2020.07.001
Wang CM, Ploia C, Anselmi F, Sarukhan A, Viola A (2014) Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J 33(12):1354–1364. https://doi.org/10.15252/embj.201386666
Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA (2018) Revisiting IL-2: biology and therapeutic prospects. Sci Immunol 3:eaat1482. https://doi.org/10.1126/sciimmunol.aat1482
Caracciolo M, Correale P, Mangano C, Foti G, Falcone C, Macheda S, Cuzzola M, Conte M, Falzea AC, Iuliano E et al (2021) Efficacy and effect of inhaled adenosine treatment in hospitalized COVID-19 patients. Front Immunol 12:34. https://doi.org/10.3389/fimmu.2021.613070
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F et al (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9(1):1123–1130. https://doi.org/10.1080/22221751.2020.1770129
Hou W, Jin YH, Kang HS, Kim BS (2014) Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J Virol 88(15):8479–8489. https://doi.org/10.1128/JVI.00724-14
Brockmann L, Soukou S, Steglich B, Czarnewski P, Zhao L, Wende S, Bedke T, Ergen C, Manthey C, Agalioti T et al (2018) Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat Commun 9(1):5457. https://doi.org/10.1038/s41467-018-07581-4
Bedke T, Muscate F, Soukou S, Gagliani N, Huber S (2019) IL-10-producing T cells and their dual functions. Semin Immunol 44:101335. https://doi.org/10.1016/j.smim.2019.101335
Gong J, Dong H, Xia SQ, Huang YZ, Wang D, Zhao Y, Liu W, Tu S, Zhang M, Wang Q (2020) Correlation analysis between disease severity and Inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. https://doi.org/10.1186/s12879-020-05242-w
Paletta-Silva R, Meyer-Fernandes JR (2012) Adenosine and immune imbalance in visceral leishmaniasis: the possible role of ectonucleotidases. J Trop Med 2012:650874. https://doi.org/10.1155/2012/650874
de Souza MC, de Assis EA, Gomes RS, Silva EDAM, Melo MN, Fietto JL, Afonso LC (2010) The influence of ecto-nucleotidases on Leishmania amazonensis infection and immune response in C57B/6 mice. Acta Trop 115(3):262–269. https://doi.org/10.1016/j.actatropica.2010.04.007
Funding
Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (GZR and MDB—CNPq proj. No 404256/2021–0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (GZR), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (GZR, ZMI), Universidade do Extremo Sul Catarinense (GZR), and Universidade Federal da Fronteira Sul (MDB, ZMI and GGO). GZR and MDB are 2 CNPq Research Fellow. MMP acknowledges grant support by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS proj. No. 20255100002535) and Marylou Ingram Scholarship.
Author information
Authors and Affiliations
Contributions
Conceptualization: MDB, ZMI, GGO. Methodology: GBS, DM, APS, GCK, MH, MMP, JDL, FM, AGB, MEDM, MDB. Investigation: GBS, DM, APS, GCK, MMP, JDL. Visualization: MDB, GGO, ZMI. Funding acquisition: MDB, GGO, ZMI, GZR, and MMP. Project administration: MDB. Supervision: MDB. Writing — original draft: GBS, DM, CEA, JLBS. Writing — review, and editing: GBS, DM, CEA, JLBS, ZMI, ZGR, GGO. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Federal University of Fronteira Sul (10/12/2020 under nº 4.333.214).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, G.B., Manica, D., da Silva, A.P. et al. High levels of extracellular ATP lead to different inflammatory responses in COVID-19 patients according to the severity. J Mol Med 100, 645–663 (2022). https://doi.org/10.1007/s00109-022-02185-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02185-4