Abstract
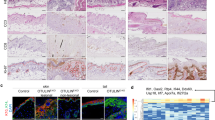
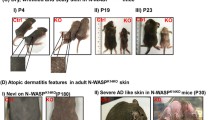
MCPIP1 (Regnase-1, encoded by the ZC3H12A gene) regulates the mRNA stability of several inflammatory cytokines. Due to the critical role of this RNA endonuclease in the suppression of inflammation, Mcpip1 deficiency in mice leads to the development of postnatal multiorgan inflammation and premature death. Here, we generated mice with conditional deletion of Mcpip1 in the epidermis (Mcpip1EKO). Mcpip1 loss in keratinocytes resulted in the upregulated expression of transcripts encoding factors related to inflammation and keratinocyte differentiation, such as IL-36α/γ cytokines, S100a8/a9 antibacterial peptides, and Sprr2d/2h proteins. Upon aging, the Mcpip1EKO mice showed impaired skin integrity that led to the progressive development of spontaneous skin pathology and systemic inflammation. Furthermore, we found that the lack of epidermal Mcpip1 expression impaired the balance of keratinocyte proliferation and differentiation. Overall, we provide evidence that keratinocyte-specific Mcpip1 activity is crucial for the maintenance of skin integrity as well as for the prevention of excessive local and systemic inflammation.
Key messages
-
Loss of murine epidermal Mcpip1 upregulates transcripts related to inflammation and keratinocyte differentiation.
-
Keratinocyte Mcpip1 function is essential to maintain the integrity of skin in adult mice.
-
Ablation of Mcpip1 in mouse epidermis leads to the development of local and systemic inflammation.






Similar content being viewed by others
References
Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ (2009) Skin immune sentinels in health and disease. Nat Rev Immunol 9:679–691
Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M (2008) Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res 49:697–714
Guttman-Yassky E, Nograles KE, Krueger JG (2011) Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. J Allergy Clin Immunol 127:1110–1118
Segre JA (2006) Epidermal barrier formation and recovery in skin disorders. J Clin Invest 116:1150–1158
van Smeden J, Bouwstra JA (2016) Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol 49:8–26
Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, Holleran WM, Uchida Y (1841) Formation and functions of the corneocyte lipid envelope (CLE). Biochim Biophys Acta 2014:314–318
Natsuga K (2014) Epidermal barriers. Cold Spring Harb Perspect Med 4:a018218–a018218
Tsuruta D, Green KJ, Getsios S, Jones JCR (2002) The barrier function of skin: how to keep a tight lid on water loss. Trends Cell Biol 12:355–357
Kobayashi T, Naik S, Nagao K (2019) Choreographing immunity in the skin epithelial barrier. Immunity 50:552–565
Fu M, Blackshear PJ (2017) RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins. Nat Rev Immunol 17:130–143
Uehata T, Akira S (1829) mRNA degradation by the endoribonuclease Regnase-1/ZC3H12a/MCPIP-1. Biochim Biophys Acta 2013:708–713
Iwasaki H, Takeuchi O, Teraguchi S, Matsushita K, Uehata T, Kuniyoshi K, Satoh T, Saitoh T, Matsushita M, Standley DM et al (2011) The IkappaB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol 12:1167–1175
Uehata T, Iwasaki H, Vandenbon A, Matsushita K, Hernandez-Cuellar E, Kuniyoshi K, Satoh T, Mino T, Suzuki Y, Standley DM et al (2013) Malt1-induced cleavage of Regnase-1 in CD4(+) helper T cells regulates immune activation. Cell. 153:1036–1049
Li M, Cao W, Liu H, Zhang W, Liu X, Cai Z, Guo J, Wang X, Hui Z, Zhang H et al (2012) MCPIP1 down-regulates IL-2 expression through an ARE-independent pathway. PLoS One 7:e49841
Mizgalska D, Wegrzyn P, Murzyn K, Kasza A, Koj A, Jura J, Jarzab B, Jura J (2009) Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. FEBS J 276:7386–7399
Monin L, Gudjonsson JE, Childs EE, Amatya N, Xing X, Verma AH, Coleman BM, Garg AV, Killeen M, Mathers A et al (2017) MCPIP1/Regnase-1 restricts IL-17A- and IL-17C-dependent skin inflammation. J Immunol 198:767–775
Ruiz-Romeu E, Ferran M, Gimenez-Arnau A, Bugara B, Lipert B, Jura J, Florencia EF, Prens EP, Celada A, Pujol RM et al (2016) MCPIP1 RNase is aberrantly distributed in psoriatic epidermis and rapidly induced by IL-17A. J Invest Dermatol 136:1599–1607
Takaishi M, Satoh T, Akira S, Sano S (2018) Regnase-1, an immunomodulator, limits the IL-36/IL-36R autostimulatory loop in keratinocytes to suppress skin inflammation. J Invest Dermatol 138:1439–1442
Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL et al (2014) Cleavage of Roquin and Regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol 15:1079–1089
Jura J, Skalniak L, Koj A (1823) Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim Biophys Acta 2012:1905–1913
Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H et al (2009) Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 458:1185–1190
Zhou Z, Miao R, Huang S, Elder B, Quinn T, Papasian CJ, Zhang J, Fan D, Chen YE, Fu M (2013) MCPIP1 deficiency in mice results in severe anemia related to autoimmune mechanisms. PLoS One 8:e82542
Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M (2010) MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med 207:2959–2973
Miao R, Huang S, Zhou Z, Quinn T, Van Treeck B, Nayyar T, Dim D, Jiang Z, Papasian CJ, Eugene Chen Y et al (2013) Targeted disruption of MCPIP1/Zc3h12a results in fatal inflammatory disease. Immunol Cell Biol 91:368–376
Swindell WR, Sarkar MK, Liang Y, Xing X, Gudjonsson JE (2016) Cross-disease transcriptomics: unique IL-17A signaling in psoriasis lesions and an autoimmune PBMC signature. J Invest Dermatol 136:1820–1830
Li Y, Huang X, Huang S, He H, Lei T, Saaoud F, Yu XQ, Melnick A, Kumar A, Papasian CJ et al (2017) Central role of myeloid MCPIP1 in protecting against LPS-induced inflammation and lung injury. Signal Transduct Target Ther 2:17066
Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P et al (2004) Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38:176–181
Anders S, Pyl PT, Huber W (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 31:166–169
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13
Hardman MJ, Sisi P, Banbury DN, Byrne C (1998) Patterned acquisition of skin barrier function during development. Development 125:1541–1552
Heyden A, Lutzow-Holm C, Clausen OP, Brandtzaeg P, Huitfeldt HS (1994) Expression of keratins K6 and K16 in regenerating mouse epidermis is less restricted by cell replication than the expression of K1 and K10. Epithelial Cell Biol 3:96–101
Stoler A, Kopan R, Duvic M, Fuchs E (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol 107:427–446
Mils V, Vincent C, Croute F, Serre G (1992) The expression of desmosomal and corneodesmosomal antigens shows specific variations during the terminal differentiation of epidermis and hair follicle epithelia. J Histochem Cytochem 40:1329–1337
Coulombe PA (1997) Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun 236:231–238
McGowan K, Coulombe PA (1998) The wound repair-associated keratins 6, 16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem 31:173–204
Liew FY, Girard J-P, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16:676–689
Vrotsos EG, Kolattukudy PE, Sugaya K (2009) MCP-1 involvement in glial differentiation of neuroprogenitor cells through APP signaling. Brain Res Bull 79:97–103
Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 283:14542–14551
Chao J, Dai X, Pena T, Doyle DA, Guenther TM, Carlson MA (2015) MCPIP1 regulates fibroblast migration in 3-D collagen matrices downstream of MAP kinases and NF-kappaB. J Invest Dermatol 135:2944–2954
Lipert B, Wegrzyn P, Sell H, Eckel J, Winiarski M, Budzynski A, Matlok M, Kotlinowski J, Ramage L, Malecki M et al (1843) Monocyte chemoattractant protein-induced protein 1 impairs adipogenesis in 3T3-L1 cells. Biochim Biophys Acta 2014:780–788
Zhussupbekova S, Sinha R, Kuo P, Lambert PF, Frazer IH, Tuong ZK (2016) A mouse model of hyperproliferative human epithelium validated by keratin profiling shows an aberrant cytoskeletal response to injury. EBioMedicine. 9:314–323
Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC et al (2015) MCPIP1 endoribonuclease activity negatively regulates interleukin-17-mediated signaling and inflammation. Immunity. 43:475–487
Schafer M, Farwanah H, Willrodt A-H, Huebner AJ, Sandhoff K, Roop D, Hohl D, Bloch W, Werner S (2012) Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol Med 4:364–379
Haines RL, Lane EB (2012) Keratins and disease at a glance. J Cell Sci 125:3923
Lessard JC, Piña-Paz S, Rotty JD, Hickerson RP, Kaspar RL, Balmain A, Coulombe PA (2013) Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc Natl Acad Sci U S A 110:19537–19542
Helenius TO, Antman CA, Asghar MN, Nystrom JH, Toivola DM (2016) Keratins are altered in intestinal disease-related stress responses. Cells. 5. https://doi.org/10.3390/cells5030035
Di Salvo E, Ventura-Spagnolo E, Casciaro M, Navarra M, Gangemi S (2018) IL-33/IL-31 axis: a potential inflammatory pathway. Mediat Inflamm 2018:3858032
Kotsiou OS, Gourgoulianis KI, Zarogiannis SG (2018) IL-33/ST2 axis in organ fibrosis. Front Immunol 9:2432
Pinto SM, Subbannayya Y, Rex DAB, Raju R, Chatterjee O, Advani J, Radhakrishnan A, Keshava Prasad TS, Wani MR, Pandey A (2018) A network map of IL-33 signaling pathway. J Cell Commun Signal 12:615–624
Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ (2013) Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol 133:2461–2470
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44:450–462
Watanabe M, Natsuga K, Nishie W, Kobayashi Y, Donati G, Suzuki S, Fujimura Y, Tsukiyama T, Ujiie H, Shinkuma S et al (2017) Type XVII collagen coordinates proliferation in the interfollicular epidermis. eLife. 6. https://doi.org/10.7554/eLife.26635
Jackow J, Schlosser A, Sormunen R, Nystrom A, Sitaru C, Tasanen K, Bruckner-Tuderman L, Franzke C-W (2016) Generation of a functional non-shedding collagen XVII mouse model: relevance of collagen XVII shedding in wound healing. J Invest Dermatol 136:516–525
Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K (1998) Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem 273:9711–9717
Nishie W (2014) Update on the pathogenesis of bullous pemphigoid: an autoantibody-mediated blistering disease targeting collagen XVII. J Dermatol Sci 73:179–186
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X et al (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 23:479–490
Talabot-Ayer D, Martin P, Vesin C, Seemayer CA, Vigne S, Gabay C, Palmer G (2015) Severe neutrophil-dominated inflammation and enhanced myelopoiesis in IL-33-overexpressing CMV/IL33 mice. J Immunol 194:750–760
Peng H, Ning H, Wang Q, Lu W, Chang Y, Wang TT, Lai J, Kolattukudy PE, Hou R, Hoft DF et al (2018) Monocyte chemotactic protein-induced protein 1 controls allergic airway inflammation by suppressing IL-5-producing TH2 cells through the Notch/Gata3 pathway. J Allergy Clin Immunol 142:582–594.e10
Acknowledgments
For the Krt14Cre mice, we are very thankful to Prof. Carien Niessen (Germany). We are grateful to the staff of the animal facility of the Faculty of Biochemistry, Biophysics and Biotechnology for help with animal breeding.
Funding
This research was supported by grants from the National Science Centre: PRELUDIUM 2014/13/N/NZ3/00729 (to P.K.) and OPUS 2016/23/B/NZ3/00792 (to J.J.). The Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Centre (KNOW) supported by the Ministry of Science and Higher Education. W.D. was supported by the Flanders Institute for Biotechnology (VIB), a UGent grant (GOA-01G01914) and by Methusalem grant (BO16/MET_V/007, Ghent University).
Author information
Authors and Affiliations
Contributions
P.K. and A.L.-C. designed and performed the experiments, and analyzed the data. P.K. performed all the animal work and histological analyses. A.P., I.R., M.K., and M.M. performed RNA sequencing. P.Kw. and J.C performed and analyzed flow cytometry data. R.P., W.S., and J.B. helped with the experiments. W.D., M.D., and L.S.-B. contributed to data interpretation. M.F. contributed mice. A.L.-C. drafted the main manuscript text; P.K. and J.J. edited it. J.J. coordinated and supervised the project. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors state no conflict of interests.
Declaration of ethics approval
These experiments are performed according to law of EU.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 814 kb)
Rights and permissions
About this article
Cite this article
Konieczny, P., Lichawska-Cieslar, A., Kwiecinska, P. et al. Keratinocyte-specific ablation of Mcpip1 impairs skin integrity and promotes local and systemic inflammation. J Mol Med 97, 1669–1684 (2019). https://doi.org/10.1007/s00109-019-01853-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01853-2